Summative Assessment Organizer part 1 of 2: Atomic Structure (ch 4)
advertisement

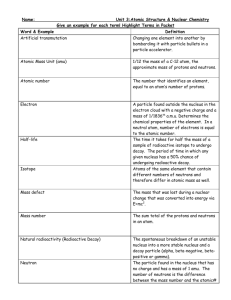
Chem F09 Unit 2: Summative Assessment Objectives: Atomic Structure and Nuclear Chemistry (ch 4 and 25) Ch 4: Atomic Structure TERMS: atom, atomic mass, atomic mass unit, atomic number, electrically neutral, electron, isotope, mass number, neutron, nucleus, proton, periodic table OBJECTIVES 1. Identify three types of subatomic particles by charge, mass, location 2. Describe the structure of atoms according to the Rutherford atomic model 3. Compare each of the following scientists concept of the atom: Dalton, Thomson, Rutherford, Bohr 4. Of the three subatomic particles, identify which determines the element and which can be lost/gained and the atom still be of the same element 5. Of the three subatomic particles: a. Name, charge, location, relative mass (in amu) b. Explain why electron’s relative mass is considered to be 0 and whether an electron is actually matter c. Since all atoms are electrically neutral, identify which subatomic particles must be in equal number in an atom 6. For ions… a. Describe a formula to calculate charge of an ion if given the counts of each subatomic particle b. Write a chemical symbol including ionic charge 7. For isotopes… a. Describe how isotopes of the same element differ from one another b. Do isotopes of the same element have the same chemical properties? c. Explain how the number of neutrons affect the mass of an atom 8. Chemical properties of an atom are mostly determined by overall charge and total number of positive subatomic particles—based on this info, explain which of the subatomic particles have the greatest affect on an atom’s chemical properties. 9. If given counts of each subatomic particle and periodic table, write a complete chemical symbol 10. If given “element name-mass number”, write a complete chemical symbol or vice versa 11. Explain how mass number differs from atomic mass in concept and in method to calculate 12. Calculate the number of neutrons in an atom 13. Calculate the atomic mass of an element if percent abundance is provided 14. Determine which isotope must have a greater percent abundance if atomic mass is provided Ch 25: Nuclear Chemistry Nuclear processes are those in which an atomic nucleus changes, including radioactive decay of naturally occurring and man-made isotopes, nuclear fission, and nuclear fusion Terms: mass defect, radioactivity, radiation, radioisotopes, alpha, beta, gamma, strong nuclear force, electrostatic force, band of stability, half life, transmutation, fission, fusion Objectives: 1. Compare and contrast nuclear reactions and chemical reactions. Make a chart listing similarities and differences between nuclear and chemical reactions. 2. Explain how any unstable nucleus releases energy 3. Mass Defect: the difference between the sums of the mass of individual particles in an atom (neglecting the electrons) compared to the actual mass of the same atom from the periodic table. The actual mass is always larger than the experimental mass whenever the nucleus contains more than one particle. The difference in mass (mass defect) is converted into energy that holds the nucleus together and can be released in nuclear reactions. Einstein’s equation, E = mc2, quantifies the amount of energy produced. E = energy (J), m = change in mass (kg), and c is the speed of light (3.0 x 10 8 m/s). 4. Compare the three main types of nuclear radiation by their composition, complete chemical symbol, charge, mass in amu, penetrating power, and shielding 5. Explain where a beta particle comes from. And it is not from the electron cloud around the nucleus. 6. Perform decay reactions for alpha, beta, and gamma radiation if given the type of decay and a starting nucleus 7. Identify the missing particle in decay reactions if given 2 of the 3 particles 8. Describe how mass and proton number of a nucleus will change after undergoing alpha, beta, and gamma decay (increase, decrease, stay the same, and by how much) 9. Compare the strong nuclear force (just called “nuclear force” in text) and the electrostatic force (aka electromagnetic force)—which stabilizes the nucleus? Which stabilizes the atom? Which destabilizes the nucleus? 10. Describe how the proton: neutron ratio changes as atomic number increases. What is significant about number 83? 11. Solve problems that involve half-life if given three of the four variables: length of half life (t1/2), elapsed time (te), initial mass (Mi) and final mass (Mf). 12. Classify nucleus as stable or unstable based on length of half life. Explain whether isotopes with long half lives or short half lives are more dangerous based on the amount of radiation emitted over time 13. What is transmutation and determine which forms nuclear reactions may cause a transmutation 14. Distinguish between fission and fusion reactions based on the number of starting and ending particles, the size of the particles used, and the amount of energy released