Significant Figures
advertisement

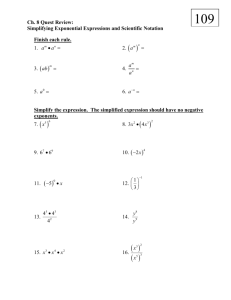
All Chemistry Sig Fig Review I. Identify how many significant figures are in the numbers below. Then round the number so that it has 3 significant figures. Next write the rounded number in scientific notation. Number # of sig. figs. rounded answer a) 1556 b) 14.532 c) 0.003728 d) 4.630 e) 174.51 f) 95450 g) 205.0 h) 60.1500 i) 0.3705001 j) 0.0003555 ________ ________ ________ ________ ________ ________ ________ ________ ________ ________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ II. scientific notation ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ Carry out the desired mathematical calculation. Give the final answer in scientific notation reflecting the correct number of significant figures. Also include the correct unit. (Remember, when adding and subtracting numbers with exponents, you must modify the numbers so that the exponents are the same.) a) 41.024 cm – 23.28 cm = ________________ k) 2.89 cm x 4.01 cm = ________________ b) 289 g – 43.7 g = _________________ l) 3.08 m x 1.2 m = ________________ c) 25.0 mL + 0.25 mL = _________________ m) 5.00 mm x 7.3216 mm = ____________ d) 1.35 x102 cm + 2.52 x101 cm = _________________ n) 2.2 x103 µm x 5.50 x10-4 µm = _______ e) 8.9 x104 m – 0.360 x103 m = _________________ o) 3.59 g / 25.00 mL = _________________ f) *200 m – 125 m = _________________ p) 0.034 x103 km2 / 17.0 x10-1 km = ______ (*not a counted number-measured with very poor precision) g) *410 nm – 23 nm = ________________ q) 2.0 cm x 4.995 cm = ________________ (*not a counted number-measured with very poor precision) h) 0.289 x106 g – 0.0437 x106 g = _________________ r) 3.008 x103 m x 1.2 m = ______________ i) 100.0 mL + 3.250 mL = _________________ s) 8.00 mm3 / 7.6 mm2 = _______________ j) 6.45 x1020 cm + 2.45 x1019 cm = _________________ t) 0.21 x1014 g / 5.00 x10-4 cm3 = ________ Answers Section I: a) 4, 1560, 1.56x103 b) 5, 14.5, 1.45 x101 c) 4, 0.00373, 3.73 x10-3 d) 4, 4.63, 4.63 x100 4 2 e) 5, 175, 1.75 x10 f) 4, 95400, 9.54 x10 g) 4, 205. , 2.05 x10 h) 6, 60.2, 6.02 x10 1 i) 7, 0.371, 3.71 x10-1 j) 4 , 0.000356, 3.56 x10 -4 a) 1.774 x101 cm b) 2.45 x102 g c) 2.52 x101 mL d) 1.60 x102 cm e) 8.9 x104 cm f) 1 x102 m 2 5 2 20 1 2 g) 3.9 x10 nm h) 2.45 x10 g i) 1.032 x10 mL j) 6.70 x10 cm k) 1.16 x10 cm l) 3.7 x100 m2 1 2 0 2 -1 1 1 2 m) 3.66 x10 mm n) 1.2 x10 µm o) 1.44 x10 g/mL p) 2.0 x10 km q) 1.0 x10 cm r) 3.6 x103 m2 0 16 3 s) 1.1 x10 mm t) 4.2 x10 g/cm 2 Section II: