quiz 3 answer
advertisement

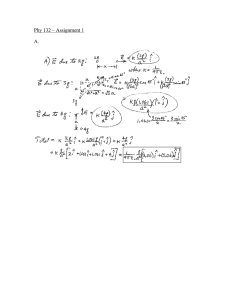
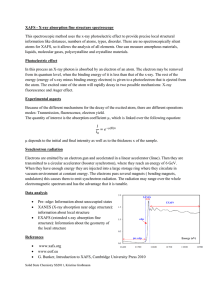
Faculty of Dentistry Faculty of Dentistry Department of Basic Sciences Quiz 3 Physics II Date: 29-5-2011 Time: 1/2h Student name ………………………… ID........................................... 1- Circle ONLY 5 correct answer: 5 marks 1 The half-life of a radioactive substance is A. half the time it takes for the entire substance to decay C. the time for radium to change into lead E. the time for half the substance to decay 2 Possible units for the disintegration constant λ are A. kg/s B. s/kg C. hour D. day−1 B. usually about 50 years D. calculated from E = mc2 E. cm−1 3 A radioactive atom X emits a β− particle. The resulting atom A. must be very reactive chemically B. has an atomic number that is one more than that of X C. has a mass number that is one less than that of X D. must be radioactive E. is the same chemical element as X 4 The most energetic continuous x-ray spectrum has an energy approximately equal to A. the energy of all the electrons in a target atom B. the kinetic energy of an incident-beam electron C. the rest energy, mc2, of an electron D. the total energy of a K-electron in the target atom E. the kinetic energy of a K-electron in the target atom 5-In connection with x-ray emission the symbol Kα refers to A. an alpha particle radiation B. an effect of the dielectric constant on energy levels C. x-ray radiation from potassium D. x-ray radiation associated with an electron going from n = ∞ to n =1 E. x-ray radiation associated with an electron going from n =2to n =1 6-In medical diagnostic applications ………………………are unwanted. A. the low energy (soft) X-rays B. the high energy (hard) X-rays C. the kinetic energy of an incident-beam electron D. none of the above . 1 Solve Only one problem 5 marks 1-The extremes of the x-ray portion of the electromagnetic spectrum range from approximately 90.0 x 10-8 m to 20.0 x 10-13 m. Find the minimum accelerating voltages required to produce wavelengths at these two extremes. eV hc o hc e1 V1 34 6.6 x10 V1 1.6 x10 19 8 x3x10 8 x90 x10 V=1.3 volt V2 34 6.6 x10 1.6 x10 19 8 x3x10 13 x 20 x10 V2= 2.4x 108 volt solution containing a radioactive isotope, which emits -particles with a half-life of 12.26 days, surrounds a Geiger counter, which records 28900 counts per hour. The back ground counting is 100 counts per hour .What counting rate will be obtained 49.04 days later? 2- A A0=28900 -100= 28800 counts per hour ln 2 ln2/(12.26)=0.056 day -1 e-t t= 49.04 day 28800 e-0.056 x49.04 A= 1848 counts per hour me=9.109 ×10−31 kg mp=1.67×10−27 kg e=1.6x10-19 C NA=6.02 x 1023 atoms/mol K=9x109 Nm2/C2 2 N= NAm / M U t c = q t V c=1/2 mv , = - N (d/dt), =B.A, R=mv/qB eV hc o = e-x F qq 1 q1q2 K 1 22 , 2 4o r r K 2 1 4o 1 = R( Z –b)2 ( 1 1 - 2) 2 m n N(t)=Noe- 3 ln 2 dN e-t = N (t) dt