Early History and Development of Atomic Theories
advertisement

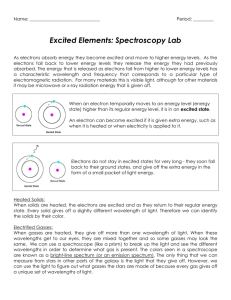
SCH3UB Sept12 Early History and Development of Atomic Theories John DALTON (1805) ________________________________________ Atom is a solid sphere Matter composed of indivisible particles (atoms) Atoms of any one element are identical to each other, different from any other element’s atoms JJ THOMSON (1897) ________________________________________ Atoms consist of negatively charged particles called electrons embedded into positive material Ernest RUTHERFORD (1911) ______________________________________ Gold foil experiment: fired positively charged alpha particles at a thin gold foil sheet; most passed straight through the foil, some were deflected at angles as they passed through and others were reflected straight back or at an angle http://www.kentchemistry.com/links/AtomicStructure/RutherfordTutorial.htm Most of the mass of an atom is concentrated in a tiny nucleus of positively charged protons and neutral neutrons both of which have approx the same mass 1 SCH3UB Sept12 Most of the atom is empty space around the nucleus occupied by very small negative electrons with negligible mass Planetary model of the atom: Neutral atoms have an equal number of + and – charges Atomic number = Z = # of protons Mass number = A = # protons + # neutrons Problems with Rutherford Model: an orbiting electron should continuously emit electromagnetic radiation (ie light) because orbiting bodies are continually accelerating; this means electrons would be losing energy and should collapse towards nucleus, but they don’t; Bohr Atomic Theory Niels BOHR (1913) Used evidence from spectroscopic analysis of different elements to deduce that electrons in an atom exist at various definite energy levels [ this is why excited electrons of a particular element always produce the same bright-line or emission spectrum which is characteristic of the element and different elements produce different coloured flames]. www.green-planet-solar-energy.com Electrons travel in the atom in circular orbits with “quantized” energy ie, only certain quantities of energy are possessed by the electrons There is a maximum # of electrons in each orbit: 2, 8, 18, etc 2 SCH3UB Sept12 An electron can be excited to a higher energy level (further from the nucleus) through the addition of electrical or heat energy When an electron falls from an excited state back to ground state, light is emitted of a certain wavelength corresponding to the energy difference between excited and ground state Skullsinthestars.com Assessment statement Describe the electromagnetic spectrum. Students should be able to identify the ultraviolet, visible and infrared regions, and to describe the variation in wavelength, frequency and energy across the spectrum. Distinguish between a continuous spectrum and a line spectrum. Continuous Spectrum:…………………………………………………………………………………. …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. Line Spectrum:………………………………………………………………………………………… …………………………………………………………………………………………………………. …………………………………………………………………………………………………………. 3 SCH3UB Sept12 Emission Line Spectrum Each element emits a different characteristic emission spectrum of coloured light that corresponds to the different quantities of energy possessed by its electrons These different colours of light / energies correspond to differences between the energy levels present in different atoms classes.mhcc.edu No two elements have the same atomic emission spectrum; the atomic emission spectrum of an element is like a fingerprint 4 SCH3UB Sept12 EMISSION SPECTRUM of HYDROGEN 2.3.3 Explain how the lines in the emission spectrum of hydrogen are related to electron energy levels. Students should be able to draw an energy level diagram, show transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references will not be assessed. Aim 7: Interactive simulations modelling the behaviour of electrons in the hydrogen atom can be used. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. When this light is passed through a prism (as shown in the figure below), four narrow bands of bright light are observed against a black background. www.horrorseek.com uwec.edu 5 SCH3UB Sept12 2.3.4 Deduce the electron arrangement for atoms and ions up to Z = 20 a) Use the handout provided of the first 20 elements on the periodic table to draw the Bohr diagrams and then write the simple electron arrangement for each neutral element. b) Write the ion that could be formed and the simple electron arrangement for each ion that could be formed by the first 20 elements. What patterns do you see? He 2 Ne 2,8 Na+ 2, 8 Ar 2,8,8 6 SCH3UB Sept12 Test Your Understanding: 1. State the electron arrangements of the following species: Si ........................................................................................................................................ P 2. 3– (a) ............................................................................................................................. ........... List the following types of electromagnetic radiation in order of increasing wavelength (shortest first). I. Yellow light II. Red light III. Infrared radiation IV. Ultraviolet radiation ..................................................................................................................................... (1) (b) Distinguish between a continuous spectrum and a line spectrum. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (1) (c) The thinning of the ozone layer increases the amount of UV-B radiation that reaches the Earth’s surface. Type of Radiation Wavelength / nm UV-A 320–380 UV-B 290–320 Based on the information in the table above explain why UV-B rays are more dangerous than UV-A. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... 4. Which statement is correct about a line emission spectrum? A. Electrons absorb energy as they move from low to high energy levels. B. Electrons absorb energy as they move from high to low energy levels. C. Electrons release energy as they move from low to high energy levels. D. Electrons release energy as they move from high to low energy levels. 5. In the emission spectrum of hydrogen, which electronic transition would produce a line in the visible region of the electromagnetic spectrum? A. n = 2 →n = 1 C. n=2→n=3 B. n=3→n=2 D. n=∞→n=1 6. What is the electron arrangement of the Mg A. 2,2 B. 2,8 2+ ion? C. D. 2,8,2 2,8,8 7 SCH3UB Sept12 8