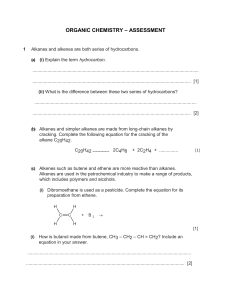
CADET COLLEGE HASAN ABDAL Chemistry Worksheet: Organic Chem Alkane + Alkene Class: 10-O-LEVEL Teacher: Mirza Saeed Ahmad Name: ----------------------------Kit no: ---------- Wing: -------- Date: 28-5-2024 PART 1: MULTIPLE CHOICE QUESTIONS (15 MCQs) Instructions: Choose the correct answer for each question. Each of one mark. 1. Alkanes are classified as saturated hydrocarbons because they contain: A) Only single bonds 2. B) At least one double bond C) At least one triple bond D) No bonds at all Which of the following best describes the general reactivity of alkanes? A) Highly reactive with most substances B) Reactive with oxygen only C) Generally unreactive except during combustion & substitution reactions 3. D) Reactive with water In a substitution reaction involving alkanes, what occurs? A) A hydrocarbon chain is broken down completely. B) An atom or group of atoms is replaced by another atom or group of atoms. C) A new alkane is formed. D) All atoms are replaced in the molecule. 4. The substitution reaction of methane & chlorine produces which of the following as a primary product? A) Chloroform 5. B) Methanol B) CnH2n B) Double covalent bond 10. B) Unsaturated hydrocarbons C) Isotopes B) Cracking C) Distillation D) Allotropes D) Filtration A) Mixing with aqueous sodium chloride B) Reaction with aqueous bromine C) Heating with carbon dioxide D) Freezing the sample In an addition reaction involving alkenes, the number of products formed is: B) Two C) Three D) Variable Which of the following is not an addition reaction of alkenes? A) With bromine 12. D) Triple covalent bond A chemical test to identify alkenes involves: A) One 11. C) Single covalent bond The process used to manufacture alkenes by breaking down larger alkane molecules is called: A) Fermentation 9. D) CnH2n-2 Alkenes are classified as: A) Saturated hydrocarbons 8. C) CnHn Methane reacting with chlorine in the presence of light forms which type of bond in the product? A) Ionic bond 7. D)Dichloromethane What is the general formula for alkanes? A) CnH2n+2 6. C) Chloromethane B) With hydrogen C) With steam D) With nitrogen The presence of a nickel catalyst is essential for alkenes' reaction with: A) Oxygen B) Hydrogen C) Nitrogen D) Carbon dioxide 13. Steam is used in the addition reaction with alkenes in the presence of: A) An acid catalyst 14. B) A base catalyst In terms of reactivity, alkenes are: 15. C) No catalyst D) A neutral catalyst A) Less reactive than alkanes B) More reactive than alkanes C) Equally reactive as alkanes D) Not reactive at all Which statement about pure hexane, C6H14, is correct? A) It boils over a range of temperatures. C) It mixes with water. B) It burns in excess oxygen to form carbon monoxide and water only. D) It melts at a fixed temperature. STRUCTURED QUESTIONS 1. Draw the structural formula for the product of a monosubstitution reaction between methane and chlorine with reaction conditions needed. [2] 2. Describe method & conditions necessary for cracking of larger alkane molecules to produce alkenes. [2] 3. Explain why aqueous bromine is used to test for the presence of unsaturation in hydrocarbons. [2] 4. Draw the structural formula for the reaction of propene with steam in the presence of an acid catalyst, and describe the product formed. [2+2]