The electron density

The electron density
The problem we now address is how to interpret the data from a diffractometer in terms of the detailed structure of a crystal. To do so, we must go beyond Bragg's law .If a unit cell contains several atoms with scattering factors and coordinates
(x j a
,y j b, z j c), then the overall amplitude of a wave
diffracted by the {hkl} planes is given by
The sum is over all the atoms in the unit cell. The quantity F hkl
is called the structure factor.
Consider the crystal shown schematically in Fig.1. The reflection corresponds to two waves from adjacent A planes, the phase difference of the waves being
2π. If there is a B atom at a fraction x of the distance between the two A planes, then it gives rise to a wave with a phase difference 2 π x relative to an A reflection. To see this conclusion, note that, if x = 0, there is no phase difference; if x = 1/2 the phase difference is π; if x = 1, the B atom lies where the lower A atom is and the phase difference is 2 π. Now consider a (200) reflection. There is now a 2 x 2 π difference between the waves from the two A layers, and if B were to lie at x = 0.5 it would give rise to a wave that differed in phase by 2 π from the wave from the upper A layer.
Thus, for a general fractional position x, the phase difference for a (200) reflection is 2 x (2 π x). For a general (h00) reflection, the phase difference is therefore h x (2 π x).
For three dimensions, this result generalizes to the equation above..
The A and B reflections interfere des tructively when the phase difference is π, and the total intensity is zero if the atoms have the same scattering power. For example, if the unit cells are cubic I with a B atom at x = y = z = 1/2, then the A,B phase difference is (h + k + l ) π. Therefore, all reflections for odd values of h + k
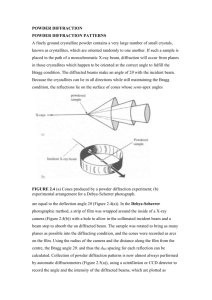
+ l vanish because the waves are displaced in phase by π. Hence the diffraction pattern for a cubic I lattice can be constructed from that for the cubic P lattice (a cubic lattice without points at the centre of its unit cells) by striking out all reflections with odd values of h + k + l. Recognition of these systematic absences in a powder spectrum immediately indicates a cubic I lattice (Fig. 2).
Fig.1 Diffraction from a crystal containing two kinds of atoms. (a) For a
(100) reflection from the A planes, there is a phase difference of 2 π between waves reflected by neighbouring planes. (b) For a (200) reflection, the phase difference is 4 π. The reflection from a B plane at a fractional distance x a from an A plane has a phase that is x times these phase differences.
Fig. 2. The powder diffraction patterns and the systematic absences of three versions of a cubic cell. Comparison of the observed pattern with patterns like these enables the unit cell to be identified. The locations of the lines give the cell dimensions.
If the amplitude of the waves scattered from A is f
A at the detector, that of the waves scattered from B is f
B e i Φ hkl , with
Φ hkl
the phase difference. The total amplitude at the detector is therefore
F hkl
= f
A
+ f
B e i Φ hkl
Because the intensity is proportional to the square modulus of the amplitude of the wave, the intensity,
I hkl the detector is
I hkl
α
F* hk l
F hk l
= ( f
A
+ f
B e - i Φ hkl ) ( f
A
+ f
B e i Φ hkl )
This expression expands to
I hkl
α f 2
A
+ f 2
B + f
A
f
B ( e - iΦ hkl + e iΦ hkl ) = f 2
A
+ f 2
B + 2 f
A
f
B cos Φ hkl
The cosine term either adds to or subtracts from f 2
A
+ f 2
B
depending on the value of Φ hkl
which in turn depends on h, k, and l and x, y, and z. Hence, there is a variation in the intensities of the lines with different hkl.
Calculate the structure factor for NaCl
Self-test Which reflections cannot be observed for a cubic I lattice?
[for h + k+ l odd, F hkl
= 0].
The intensity of the (hkI) reflection is proportional to IF hkl l 2 , so in principle we can determine the structure factors experimentally by taking the square root of the corresponding intensities . Then, once we know all the structure factors F hkl
, we can calculate the electron density distribution, Ρ(r), in the unit cell by using the expression
where V is the volume of the unit cell. This equation is called a Fourier synthesis of the electron density.
Calculating an electron density by Fourier synthesis
Answer
The results are plotted in Fig. 3 (blue line). The positions of three atoms can be discerned very readily. The more terms there are included, the more accurate the density plot. Terms corresponding to high values of h (short wavelength cosine terms in the sum) account for the finer details of the electron density; low values of h account for the broad features.
Fig. 3 The plot of the electron density calculated in The last example (blue) and the Self-test below (purple).
Self-test Use mathematical software to experiment with different structure factors (including changing signs as well as amplitudes). For example, use the same values of F h as above, but with positive signs for h ≥ 6.