Section 11.5

The Formula for a
Hydrate
Chemistry 11.5
Objectives
Explain what a hydrate is and how its name reflects is composition.
Determine the formula for a hydrate.
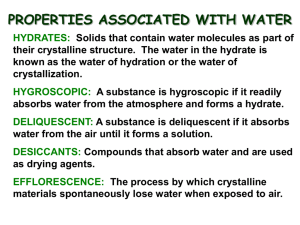
Hydrate
Key Terms
Naming Hydrates
A compound that has a specific number of water molecules bound to its atoms.
The number of water molecules is written following a dot.
Na
2
CO
3
• 10 H
2
O sodium carbonate decahydrate (Table 11-1)
Note: When calculating molar mass, the mass of water associated with the formula must be included.
Analyzing a Hydrate
To analyze a hydrate, the water associated with the compound must be driven off.
Usually this is done by heating the material.
The remaining substance is termed anhydrous:
“without water.”
Uses of Hydrates
Absorb water when in the anhydrous form.
Added to fuel to keep water out the of mixture.
Keeps electronics safe from humid air conditions.
Formula for a Hydrate
Determine the number of moles of water associated with one mole of the hydrate.
Example: Suppose you have a 5.00 g sample of a hydrate of barium chloride.
Formula is BaCl
2
• x H
2
O.
After heating substance until water is driven off, mass is 4.26 g.
Formula for a Hydrate
5.00 g BaCl
= 0.74 g H
2
2 hydrate – 4.26 g anhydrous BaCl
O.
2
Now convert the grams to mol.
4.26 g BaCl
2 x 1 mol BaCl
2
= 0.0205 mol BaCl
2
208.23 g BaCl
2
0.74 g H
2
O x 1 mol H
2
O = 0.041 mol H
2
O
18.02 g H
2
O
Formula for a Hydrate
To determine the coefficient that precedes water in the formula for a hydrate:
(moles water/moles compound)
0.041 mol H
2
O = 2
0.0205 mol BaCl
2
BaCl
2
• 2 H
2
O
Pg 340
Practice Problems
Homework
Section 11.5 Problems 33 and 34 on page 877