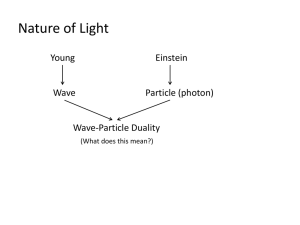
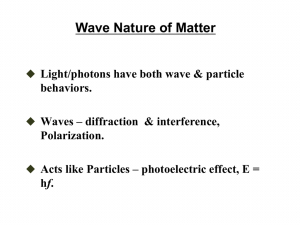
SW2: Scientific Revolution: Quantum Mechanics Any commonsense
advertisement

SW2: Scientific Revolution: Quantum Mechanics Any commonsense model of the atom is destined to fail. In static models, the atom collapses due to the electrostatic force of attraction the nucleus exerts on the electrons. Dynamic models, like the planetary model, also fail because the atom loses energy as the accelerating electrons emit EM waves, again collapsing the atom. We need a model in which the electron is somehow dynamic (orbiting) but at the same time static (not emitting EM waves)— something physicists call a stationary model. For example, a perfectly smooth spinning top is dynamic (rotating), but appears to be static—you can’t tell that it’s spinning because nothing is changing; it always looks the same. Part A: The Rotating Ring The electron cannot orbit around the nucleus as a point-like particle. What if we spread the mass and charge of the electron out into a rotating ring? 1. A rotating ring of charge behaves like a current-carrying wire. Would the rotating ring emit EM waves? Why or why not? 2. Consider the electrostatic forces acting inside the ring. Would such a structure be stable? Why or why not? Would we be able to observe it? Part B: Standing Waves The rotating ring idea is on the right track, but we have never observed such rings. We always “see” electrons as point-like particles. In preparation for Part C we will need to review some facts about waves: (1) A wave can be in many places at the same time, and (2) Two waves can exist simultaneously in the same place. 1. Stretch a coiled spring (e.g. a Slinky) between two people on a smooth, horizontal surface (hard floor or table). Wiggle one end of the spring at a constant rate. Where is the wave? What is the direction of the wave? 2. Wiggle both ends of the spring at the same rate. This creates two waves travelling in opposite directions along the spring, existing simultaneously in the same place. Adjust the rate until you get a stable pattern. Notice that the combined wave is not travelling in either direction. It is a wave—it oscillates side to side— but it is not travelling. This is called a standing wave. What happens to the standing wave as you gradually increase the frequency of vibration? Can you create standing waves at higher frequencies of vibration? Part C: The Quantum Model In the quantum model of the atom, the electron is a point-like particle whose behaviour is described by a wave. If the wave is moving, the electron is moving. Wherever the wave exists, the electron can potentially exist. The weird thing is that the electron does not exist at any definite location until its location is measured. Left undisturbed, the electron behaves as if it is spread out like a wave, and stationary states similar to the rotating ring become possible. Use this simulation (http://www.falstad.com/qmatom/) to visualize these waves. Note that these waves are mathematical—the electron’s mass and charge are not physically spread out. 1. Start the simulation. In the top-right drop down menu select “Complex Combos (n=1-4)”. Click on “Clear” then move your mouse over the little circles in the bottom-left panel, noting the yellow text that appears just above the panel. Click on the “n=2, l=1, m= –1” circle, which is the top circle in the second column. Finally, rotate the view by clicking on the z-axis in the top right corner of the main panel and dragging it down until the z disappears at the origin and the y-axis points straight up. This is a “top down” view of a single electron “orbiting” the nucleus of a Hydrogen atom. (The nucleus is at the centre, but not shown.) What do you see? 2. The colours represent the “phase” of a donut-shaped wave circulating around the nucleus, showing that the wave “crests” and “troughs” are moving. Observe that the moving electron is behaving as if it is in two places at once—actually everywhere at once, wherever the wave is non-zero! Select the “View” drop down menu from the top menu bar and deselect “Phase as Color.” You will now see a probability pattern: the probability, at any instant of time, of finding the electron at various locations around the nucleus. In what way is the electron static? In what way is it dynamic? Do you think the electron is emitting EM waves? Draw comparisons with the rotating ring in Part A. 3. Reselect “Phase as Color,” click on “Clear,” and then choose the “n=2, l=1, m=+1” circle. Note the direction of rotation of this wave. Now click on the “n=2, l=1, m=–1” circle. You have just combined two waves circulating in opposite directions around the nucleus to produce a standing wave. This standing wave describes an electron behaving as if it is moving both clockwise and counterclockwise at the same time! Is this electron “moving”? Click on the x-y-z coordinate system and rotate it to view this standing wave from different angles. Deselect “Phase as Color” to reveal the corresponding probability pattern. In what way is the electron static? In what way is it dynamic? Do you think the electron is emitting EM waves? Why or why not. By describing the behavior of a particle using a wave, anything a wave can do a particle can do. A wave can be in many places at once, or be moving in different directions at once—so can a particle! This leads to very noncommonsensical behaviour of electrons inside atoms, and yet these are the lengths scientists have gone to in order to construct a working model of the atom—one that allows us to understand how atoms can exist in our universe.