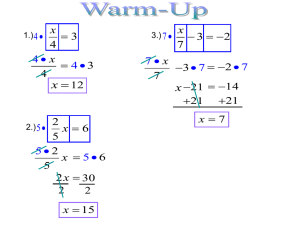CHEMISTRY 1411 LABORATORY EXPERIMENT 4
advertisement

63 Determining the Percent Composition of Ammonium Chloride, Salt, and Sand in a Mixture PRE-LAB ASSIGNMENTS: To be assigned by your lab instructor. STUDENT LEARNING OUTCOMES: By the end of this lab, the student should be able to calculate the percent composition of a mixture. The student should understand the concept of determining the mass of a substance by taking the difference of two other masses. Learn how to use physical properties to separate the components of a mixture. EXPERIMENTAL GOALS: The purpose of this procedure is to determine the percent composition of an unknown mixture that contains ammonium chloride (NH4Cl), salt (NaCl), and sand, [(SiO2)n]. INTRODUCTION: This lab is the first of a series of procedures which are designed to introduce the concept of stoichiometry, the study of the numerical relationships in chemical formulas and reactions. In this procedure, we will be dealing with a mixture of three compounds: ammonium chloride, salt, and sand. These compounds will be separated and weighed by exploiting differences in their physical properties. In the next experiment (Determining the Percent Composition of Potassium Chlorate in a Mixture), we will perform a similar analysis, but instead we will exploit a difference in the chemical properties of the substances being analyzed. Most solid substances, when heated, undergo a phase transition from the solid phase to the liquid phase, a process known as melting. However, some substances go from the solid phase directly to the gas phase, a process known as sublimation. (A familiar example is frozen carbon dioxide, or dry ice. If you put a chunk of dry ice on a tabletop, the dry ice does not melt, but the chunk gets smaller and smaller as the particles in the solid warm up and go directly into the gas phase.) Ammonium chloride sublimes when heated, which gives us a convenient way to separate ammonium chloride from salt and sand. By knowing the weight of the sample before and after heating, the mass of the ammonium chloride in the sample can be determined. Another physical property can be exploited to separate the sodium chloride from the sand: sodium chloride is soluble in water, while sand is not. (As you know if you’ve ever been to a beach.) Hot water will be added to the mixture to dissolve the sodium chloride, and the salt 64 water is removed by decanting it away from the sand. The sand is then dried over a low flame so that its mass can be determined. Once the weights of each component are known, the percent composition of the mixture is determined by dividing the weight of each component by the total weight of the sample. PROCEDURE: A. Weighing the Unknown Sample. 1. Obtain a sample of an unknown mixture of ammonium chloride and sand from the stockroom and record the sample number on the lab report (1).1 2. Clean an evaporating dish, warm it over a Bunsen burner to dry, allow it to cool, and weigh it to the accuracy of the balance (3). Handle the clean dish with tongs, not your hands. Add between four and five grams of your unknown mixture to the dish, weigh it again (2), and calculate the mass of the unknown mixture (4). B. Separation of Ammonium Chloride from the Mixture by Sublimation. 3. Place the evaporating dish containing the mixture on a wire gauze held by an iron ring in the fume hood (see Figure 1.) Heat carefully at first to avoid any spattering, and increase the heat after a couple of minutes. After heating strongly for 10 minutes, remove the flame and stir the contents with a glass stirring rod. Heat the sample again for another 10 minutes, or until no more white fumes are observed. Using your crucible tongs, carefully move the hot evaporating dish to a wire gauze or a clay triangle bent into the shape of a tripod, and allow the dish and its contents to cool in the hood. Do NOT put the hot dish down directly on the floor of the hood or the lab bench, because when the dish cools off, it may stick. 4. After the dish has cooled to room temperature, weigh the dish, salt and sand (5). Figure 1. Apparatus for the sublimation of ammonium chloride. 1 Number in parentheses refer to the line in the lab report where this information should be recorded. 65 Figure 2. Removing sodium chloride by dissolving it in water. C. Separation the Salt from the Mixture. 5. Place a piece of filter paper in the shape of a cone in a glass funnel and set that in an Erlenmeyer flask, as shown in Figure 2. Heat 200 mL of distilled water to boiling. 6. Add enough of this hot distilled water to your sand/salt mixture to fill the evaporating dish about 2/3rds of the way, and stir with a glass stirring rod. Let the mixture settle, then decant (pour off) the salt water solution through the filter paper in the funnel. Try to avoid pouring the sand out of the evaporating dish. Add another portion of hot water, stir, allow it to settle, and decant the liquid. Repeat this process until all of the hot water has been used. 7. If any sand was poured onto the filter paper, return it to the evaporating dish using the wash bottle provided, and a minimum amount of water. The salt water may be discarded. D. Drying and Weighing the Sand. 8. Position the evaporating dish containing the wet sand about 10-15 cm above the tip of the Bunsen burner flame to avoid loss of material by spattering. Heat the sand in the evaporating dish gently to evaporate the water remaining in the sand. As the sand approaches dryness, gently shake the dish to cause the sand to loosen and slide around, then heat strongly for five minutes. 9. Allow the dish and contents to cool, and weigh them (7). Re-heat vigorously for five minutes, allow dish and contents to cool, and re-weigh (8). If this weight agrees with that recorded in (7), the sample is dry. If not, continue the process until there is no further weight loss. 10. Clean off the desk with wet paper towel at the end of the lab period. 66 CALCULATIONS: 11. Determine the weight of the unknown mixture (4), the ammonium chloride (6), the sand (9), and the salt (10). 12. Calculate the percentage of each component (C) in the mixture (8) using the formula weight % C grams C 100 total grams sample 67 LAB REPORT Determining the Percent Composition of Ammonium Chloride in a Mixture Name ________________________________ Date _________ Partner ________________________________ Section _________ Report Grade ______ 1. Unknown number ________________ 2. Weight of unknown mixture and container __________ 3. Weight of empty container __________ 4. WEIGHT OF UNKNOWN MIXTURE __________ 5. Weight of sand, salt and container (after sublimation) __________ 6. WEIGHT OF AMMONIUM CHLORIDE __________ 7. Weight of dry sand and container (after first heating) __________ 8. Weight of dry sand and container (after second heating) __________ 9. WEIGHT OF SAND __________ 10. WEIGHT OF SALT __________ % ammonium chloride __________ SHOW CALCULATIONS: wt. salt % ammonium chloride % sand % salt % sand __________ % salt __________ 68 QUESTIONS: 1. You may notice that your percentages do not necessary add up to exactly 100%. What accounts for this result? 2. Ammonium chloride is also soluble in water. Why would we not be able to remove the ammonium chloride from the mixture by dissolving it in water? 3. We determined the mass of the sodium chloride by difference. How could we determine its mass directly?