van_allende01 - Consortium for Advanced Radiation Sources
advertisement

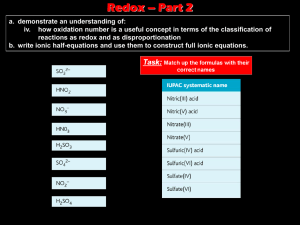
VANADIUM OXIDATION STATES IN ALLENDE CAI FASSAITE AND SYNTHETIC PYROXENE. S.R. Sutton1,2, S. Simon1, L. Grossman1, J.S. Delaney3, J. Beckett4, M. Newville2, P. Eng2, and M. Rivers1,2. 1Department of Geophysical Sciences and 2Consortium for Advanced Radiation Sources, The University of Chicago, Chicago, IL 60637 (sutton@cars.uchicago.edu); 3Geology Department, Rutgers University, New Brunswick, NJ; 4Geological & Planetary Sciences, Caltech, Pasadena, CA 91125 [More sample description here?] The microXANES analyses were performed with the GeoSoilEnviroCARS x-ray microprobe at the Advanced Photon Source (APS, Argonne National Laboratory, IL). The apparatus consisted of an APS undulator x-ray source, silicon (111) cryogenic monochromator, Kirkpatrick-Baez microfocusing mirrors (ref) and a germanium solid state x-ray fluorescence detector. The x-ray beam size was 5 m. XANES spectra were typically measured over the V K absorption edge (5465 eV) from 5450 to 5500 in 0.2 eV steps (~10 sec dwell) with additional measurements made well below (5400 eV) and well above (5700 eV) the absorption edge for normalization purposes (absorption step defined to be 1000). The pre-edge peak (near 5470 eV) was characterized by the energy and normalized intensity at the maximum. Previous work has shown these parameters to be useful for oxidation state determinations. Energy calibration was accomplished using a V metal foil spectrum and assigning the first inflection point to be 5465 eV. Standardization in terms of preedge peak energy and intensity versus oxidation state was accomplished using glasses characterized by optical spectrometry (H. Schreiber, private communication) and measurements on oxides (Wong et al. 1984). In addition, spectra were obtained on 2 pyroxene crystallization experiment charges produced by J. Beckett (Caltech) at the C-CO buffer (CMAS @ 1280C; log(fO2) ~= -17). Results: A scan of V concentration versus position was done (2 m step) across the “V spike” observed previously near analysis spot 5. This scan showed the presence of the narrow (<~10 m), high-vanadium zone. The concentration “step” of about 10:1 is comparable to that observed in electron microprobe data. Allende Fassaite Vanadium XANES: XANES spectra were obtained as spot analyses at pre-selected positions near those chosen for electron microprobe analysis. Qualitatively, the fassaite spectra were similar exhibiting very small pre-edge peaks indicative of a highly reduced oxidation state. The pre-edge peak intensi- Pre-edge Peak Energy (eV rel. 5465) Introduction: Refractory inclusions in carbonaceous chondrite meteorites contain cosmochemical clues about the processes at work in the early evolution of the Solar System. Some refractory inclusions may be direct gas-to-solid condensates, but many underwent melting as individual bodies in the nebula. A major mineral in one of the dominant types of refractory inclusions is fassaite, a Ti-, Al-rich clinopyroxene. Many fassaite grains are stongly zoned in Ti and V abundance with abundances decreasing from core to rim and concentration spikes are common. These two elements are also the only major elements in this phase with multivalent chemical characteristics. One possibility is that the zonation resulted from variable redox conditions at the time of crystallization. This interpretation is particularly compelling for titanium because Ti3+ is known to be more compatible in fassaite than Ti4+(ref). In fact, the Ti3+/Ti4+ ratio in these fassaites has been shown by stoichiometric treatment of electron microprobe data to increase at the abundance spikes (ref). We report here on our initial results using x-ray absorption near edge structure (XANES) spectroscopy (refs) for determining the oxidation state of vanadium with ~ micrometer spatial resolution. Measurements were conducted both on Allende fassaite and synthetic pyroxene. The ultimate goal is to place constraints on the oxygen fugacity of the solar nebula during the crystallization of individual fassaite grains by also determining the pyroxene/liquid partitioning behavior of V as a function of valence state. Methods: The Allende fassaite studied here was grain ZF2 on thin section TS34 described previously (ref). 6 Schreiber Glass Wong 1984 Oxides Allende Fassaite Beckett pyroxene 1c Beckett melilite 1c 5 4 3 2 2 3 4 5 V Oxidation State Figure 2: Peak energies vs oxidation state. Oxidation states for the Allende and Beckett samples are those determined using the intensities (figure 1). SHORT TITLE HERE: A. B. Author and C. D. Author ties fall between those of V3+ dominated oxides (e.g., Schreiber 112 and 113) and V2+ oxides. The latter are expected to show no pre-edge intensity (as in VO) due to the expected high symmetry of the octahedral site. Thus, the presence of a pre-edge peak indicates vanadium in the fassaite is not totally divalent. XANES spectra can also be sensitive to crystallo- 140 Schreiber Glass Pre-edge Peak Intensity 120 Oxides 100 80 Allende Fassaite 60 40 Beckett pyroxene 1c 20 0 2 2.5 3 3.5 Beckett melilite 1c V Oxidation State Figure 1: Pre-edge peak intensity versus V oxidation state showing V2+-V3+ region only. graphic orientation relative to the polarization direction of the synchrotron radiation, but this effect is expected to be less important as site symmetry increases. To check for this effect, the two spot analyses were repeated with the thin section rotated 90 degrees. These spectra were identical to those obtained initially indicating negligible orientation effect. Quantitative oxidation states were computed from the pre-edge peak intensities using a linear interpolation between the intensity measured for Schreiber glass 112 (oxidation state determined by optical spectrometry of 3.12) and zero (expected for V2+). The results are shown graphically in figure 2. The data in figure 2 is expanded in figure 3 to show only V2+-V3+ space. The mean V oxidation state for the fassaite measurements is 2.4 with a standard deviation of 0.1. The small standard deviation indicates that the large V concentration variation in this grain (order of magnitude) is unlikely to be due to variations in V redox state. The fairly tight range of intensity values is comparable to the typical measurement precision. The variation in oxidation state along the traverse is uncorrelated with vanadium content (figure 4). Synthetic Materials: Beckett’s samples VT01-1a (rapid quench) and VT01-1c (cooled at 50 deg/hr to 1000) were measured. Both showed well developed crystals and XANES spectra were obtained on grains identified beforehand by Simon. These results are also shown on figures 2 and 3. The pyroxene grain noted on Steve’s VT01-1c BSE photo yielded an oxidation state indistguishable from the mean of the Allende fassaite measurements (2.4). Two other phases were analyzed, melilite (2.4) and a dark-gray (BSE) phase tentatively denoted “glass” (2.7). Together these results suggest the pyroxene crystallation experiments reproduced the crystallization conditions of the Allende fassaite grain fairly closely. Beckett’s sample VT01-1a was very interesting … and puzzling. A line scan of the V content was done across a region that included pyroxene, melilite and glass, and the locations of the grain boundaries were easily recognized by steps in the V K fluorescence intensity profile. However, the XANES spectrum for each of these regions was the same and consistent with glass spectra we’ve obtained previously on the reduced glasses from the Hanson, Schreiber and Canil synthetic suites. The only interpretation that comes to mind at the moment is that the grains contain melt inclusions. Such inclusions are not obvious viewing in the petrographic scope…if they’re there, they’re likely to be tiny (sub micron?). The pre-edge intensity for these spectra yielded an oxidation state of 3.2, i.e., significantly more oxidized than the other measurements. This sample needs to be investigated further. No XANES measurements were done on sample VT01-1b that appeared to have no crystals (Simon’s observations). Figure 5 shows the pre-edge peak energies plotted versus oxidation state (determined from intensities). This plot shows that the measured energies for the pyroxenes are consistent with the oxidation state assignments based on the intensities. A reasonably good correlation exists between the pyroxenes, the Schreiber glasses and the oxides measured by Wong 1984. Discussion: The oxidation state standards used in this work are a FAD glass containing predominately V3+ and a V2+ oxide (VO). An underlying assumption is that the site geometry is the same in the standard and the unknown. This is likely to be a good assumption here because octahedral coordination of V dominates. VO consists of regular octahedral VO6 units. Optical spectroscopy on V3+ in albite-diopside glass shows vanadium in octahedral sites (Keppler 1992). In diopside, based on ionic size arguments, V3+ is likely to go into the M1 octahedral site but V2+ is large enough that it may be forced into the M2 site substituting for Ca2+. The coordination of V2+ in M2 is uncertain. Calcium is 8-fold but divalent cations that substitute for it (e.g., Mn2+ and Fe2+) are octahedral. The coordination geometry of V in these samples needs to be investigated further perhaps using extended x-ray absorption fine structure spectroscopy. References: Delaney, J.S., Jones J.H., Sutton S.R., Simon S. and Grossman L. (1999) In situ microanalysis of vanadium, chromium, and iron oxidation states in extraterrestrial samples by synchrotron microXANES (SMX) spectroscopy. Meteoritics & Planet. Sci. 34, A32. Delaney J.S., Sutton S.R., Newville M., Jones J.H., Hanson B., Dyar M.D. and Schreiber H. (2000) Synchrotron micro-XANES measurements of vanadium oxidation state in glsses as a function of oxygen fugacity: experimental calibration of data relevant to partition coefficient determination. Lunar Planet. Sci. XXXI, abstr. # 1806 (CD-ROM). Quartieri, S., G. Antonioli, G. Artioli, and P. Lottici (1993) XANES study of titanium coordinatioin in natural diopsidic pyroxenes. Eur. J. Mineral. 5, 1101-1109. Simon S.B. and Grossman L. (1991) Profiles of Ti3+/Titot ratios in zoned fassaite in Allende refractory inclusions. Meteoritics 26, 395. Simon S.B., Davis A.M. and Grossman L. (1992) Evidence for changes in redox state during crystallization of Allende type B1 inclusions. Meteoritics 27, 289-290. Waychunas, G. (1987) Synchrotron radiation XANNES spectroscopy of Ti in minerals: Effects of Ti bonding distances, Ti valence and site geometry on absorption edge structure. Am. Mineral. 72, 89-101.