Notes on Density
advertisement

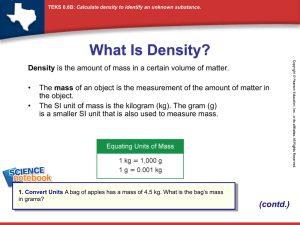
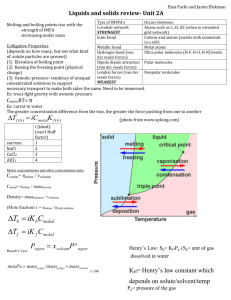
Science Study Notes DENSITY DENSITY – how heavy something is for its size The amount of matter compared to the amount of space it occupies The amount of atoms in a cubic centimeter Low Density – the atoms are spread far apart from each other High Density – the atoms are very close to one another Note* Different materials have different densities as different kinds of atoms line up differently In our experiment ( 4 metal bars) the different bars had different masses , even thought they had the same volume (size) We are able to deduct that density is the amount of matter (numbers of atoms) compared to the space it occupies We know that objects made of different materials may have different densities…BUT… how can we measure density? To measure density, we need to know 2 things: volume and mass We use a formula to find density: Density = mass Volume Also…think UNITS: example: One of our blocks had a mass of 27.3 g and a volume of 10cubic cm The amount of matter The space it occupies Mass = 27.2g Volume 10 cubic cm Density = 2.72 g/cubic cm Say a substance has a mass of 100 g and a volume of 10 cubic cm. What is its Density? The amount of matter The space it occupies Q. Mass = 100g Volume 10 cubic cm (10 grams per cubic centimeter) What happens to the density if we cut the substance in half? The amount of matter The space it occupies Mass = 50 g Volume 5 cubic cm (10 grams per cubic centimeter) Answer: Nothing…the density remains the same regardless of the size of the substance NOTE*** The density of a pure substance is always the same regardless of the size of the sample Therefore… Density is a characteristic property Characteristic Property = An identifying trait i.e. you can identify an unknown material by finding its density - (need mass and volume) A Characteristic Property doesn’t change regardless of the amount or size of the sample. Water has a density of 1.0 whether you measure a drop, a capful or a swimming pool full. Ultimate characteristic properties: your fingerprint, your DNA Every pure material has a specific density The density of a pure substance at room temperature is always the same Example Water has a density of about 1.0 g/mL whether it is a drop, o a cup, or a swimming pool full Note* When you find density, you are actually finding the number of atoms in 1 cubic centimeter See text – p 14 re: Layers and Density Layers are formed according to the densities of the liquids. The heaviest will drop to the bottom of the container, with the next heaviest layer forming on top of that, and the lightest layer forming at the top FORMULAS Formula – used to show the relationship between variables Ex. D = M V shows the relationship between density, mass and volume (3 variables) EX. A metal block has a mass of 100g and a volume of 10 cubic centimeters. What is its density? D = M V 100g + 10 grams/cubic centimeters 10 cc Q. What happens if I cut the block in half? What will happen to the density? M = 50g V = 5 cc A. D= M V 50g 5 cc = 10 g/cc Nothing changes – the density of a substance remains the same regardless of how much or how little the sample is. This is the formula for density, but from this formula we can find the formula for finding mass and volume We must rearrange the formula to find mass or volume. There are 4 ways to re-arrange a formula: 1. Algebra 2. Units 3. Triangle 4. Memorize the formula (See notes on using the algebra, units and triangle methods) OR….MEMORIZE! D= M V M= D x V V = M D NOTES ON SOLUBILITY TERMS: Solubility – how well one substance dissolves in another Solute – the chemical which is being dissolved Solvent – the chemical which dissolves the other chemical Example: Making hot tea – the tea leaves are the solvent and the hot water is the solute Making chocolate milk - the chocolate syrup is the solvent and the mile is the solute (If liquids are miscible (soluble) with each other, they simply dissolve and mix together