1.05 Guided Notes
advertisement

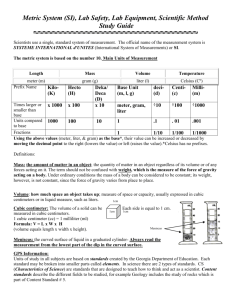
1.05 Matter and Measurement My Goals for this Lesson: Describe matter and its characteristics. Identify the units of measurement for matter. I’m preparing to understand matter and the interaction of matter and energy. What: Learn About the properties of matter in all three tabs Matter occupies space, is called ________ and can be measured in cubic centimeters, cm 3, or liters, L. We can measure the volume of a solid, liquid, or gas. The more matter there is in an object, the greater its _______. It is measured using a tool called a balance. Weight is a measure of the Earth’s gravitational attraction for matter. Mass is NOT affect by gravity. The terms mass and weight are used interchangeably in everyday life, but they mean very different things in science. Why: Measurements: Present all measurements with the number and the _____. A common system of measurements helps scientists share and evaluate data easier. The system of measurement that is used by scientists is called ____________. View the volume, mass, length, density tabs One way that scientists measure volume in milliliters or liters is using a __________, a tall, narrow, transparent, container that helps give a more accurate measurement. We will be measuring ______ instead of weight in this course, using a _______. There are two common types of balances triple beam balances and top loaded digital. The SI system uses length measurements that are all derived from the _________. Density is the _______ of a substance per a certain unit of _________. Watch the density video: http://player.discoveryeducation.com/index.cfm?guidAssetId=886A0ECD-33AC-4A5BA184-FB5C91FE2A07 Each kind of _______ has its own density, to calculate density we must know the volume and mass of the object. Density is equal to the ______ over the _______. The mass of something is found with a tool called _______. The volume may be a little more challenging to find: If the object is shaped like a box with straight sides and edges, then we simply measure the _____, _____, and _____ of the object. Then we multiply the three numbers times each other to get the volume, in cubic units. The formula is V=LxWxH For an object with an odd shape, we can use a special method called ______ __________. An object submerged in water will displace or push aside an amount of water equal to _________ of the object. So we can measure the volume of this rock by first recording a starting amount of water in the graduate cylinder. Then when the rock is lowered into the graduate cylinder, the water will rise. The new level is recorded and the difference between the two water levels is compared. Subtract the starting level from the final level to get the volume of the submerged object. Every milliliter of water that rises is equal to one cubic centimeter of volume. Example: The density of a piece of wood can be determined by measuring the mass and volume of the wood. The mass is ten grams. Each side of the cube of wood is 2.5 centimeters, so here is the calculation: 2.5 times 2.5 times 2.5 equals 15.625 cubic centimeters. Density is equal to mass over volume. The mass is 10 grams and the volume is 15.625 cubic centimeters. So the density of the wood is calculated by dividing 10 grams by 15.625 cubic centimeters for its density: .64 grams per cubic centimeter. Objects with densities greater than water _______ and objects with densities ______ than water float.