Not all crystals are created equal
advertisement
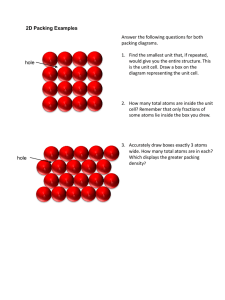
NOT ALL CRYSTALS ARE CREATED EQUAL: A HANDS-ON INVESTIGATION OF UNIT CELL STRUCTURES C Susanne M. Dana Blacksburg High School 2013 VMI STEM Education Conference: “Science with the future in mind” October 8, 2013 Why study crystals? • Synthesis of several areas of STEM • Modeling • Symmetry and Geometry • Periodic trends • Connection of microscopic structure to macroscopic properties • Current applications • Solid-state chemistry and physics not emphasized in traditional high school programs • Manipulation of composition and structure creates desirable properties • Gemstones • Steel • semiconductors Self-Assembly Activity • RULES: • You must hold hands. No hand can be left untouched. • Your right hand must touch someone else’s right hand and your left hand must touch someone else’s left hand. • You cannot cross your arms. • RESULTS: A complete circle with each participant alternating the direction they are facing. What is a unit cell? •Single unit from which structure of compound can be predicted •Modeling in 2-D •Scrapbook paper, wallpaper, etc. •Corner/vertex, edge Investigating unit cells in 3-D • Different types • Simple cubic • Face-centered cubic • Body-centered cubic • Investigation • Forming cell • Analyzing for • # atoms per unit cell • Coordination number • Connection between edge length and radius of atom • Connection to density, Avogadro’s number, molar mass Simple Cubic • Making a unit cell • Use template A and block with half moon • Align template on block and put rods in four corners of shaded area • Place two clear atoms on each rod • One unit cell! Expand pattern • Investigating… • What portion of each atom is within each unit cell? • How many atoms are in a unit cell? • What is the relationship between edge length and atomic radius Geometry Applying to a problem… • Polonium (Po) is one of the few metals that crystallizes in a simple cubic arrangement. It has an edge length of 334 pm. • What percentage of the unit cell is taken up by atoms? Face-Centered Cubic • Making a unit cell • Use template C and block with half moon • Align template on block and put nine rods in corners, edges, and center of shaded area • Place one clear atom in each corner and center (like an x) • Place one on each edge (four total) • Repeat corner and center • One unit cell! Expand pattern • Investigating… • What portion of each atom is within each unit cell? • How many atoms are in a unit cell? • What is the relationship between edge length and atomic radius? Geometry Applying to a problem… • The density of an unknown metal is 2.64 g/cm3 and its atomic radius is 215 pm. It has a face-centered cubic lattice. • What is the edge length? • What is the volume of a unit cell? • What is the molar mass of the metal? • Identify the metal. Body-Centered Cubic • Making a unit cell • Use template F and block with full circle • Align template on block and put five rods in shaded area (corners and center) • Place one clear atom in each corner • Place one in center • Repeat corner layer • One unit cell! Expand pattern • Investigating… • What portion of each atom is within each unit cell? • How many atoms are in a unit cell? • What is the relationship between edge length and atomic radius? Geometry Applying to a problem… • Sodium has a density of 0.971 g/cm3 and crystallizes with a body-centered cubic unit cell. • What is the edge length of the cell in picometers? • What is the radius of a sodium atom in picometers? • What percent of the unit cell is taken up by sodium atoms? Computer tutorial • http://www.wwnorton.com/college/chemistry/gilbert2/chemtours.asp • Chapter 11: Unit Cell • Section 5: What are the three common cubic systems? Name and draw a rough sketch for each one. • Section 6: What are the four different lattice positions an atom can occupy? How many unit cells share each one? • Continue through the tutorial and complete questions 1-4 at the end. Ionic compounds… •CsCl – simple cubic •NaCl – face centered cubic Other ideas •Crystal lab…integrates several areas of chemistry (in packet) •Rock Candy •Bragg equation Bragg’s Law For more information… • Self assembly games: • http://pbskids.org/dragonflytv/pdf/DragonflyTV_SelfAssemblyGames.pdf • Institute for chemical education (unit cell kits) • http://ice.chem.wisc.edu/Catalog/SciKits.html#Anchor-Solid-31140 • Pics of geometries…thanks to • http://penyayangbercahaya.wordpress.com/2010/03/09/packing-efficiency/ • Bragg’s law pic…thanks to • http://www-outreach.phy.cam.ac.uk/camphy/xraydiffraction/xraydiffraction7_1.htm