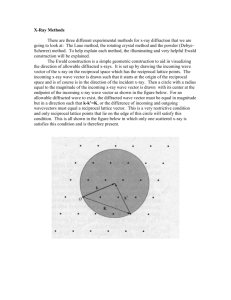
Homework 2
advertisement
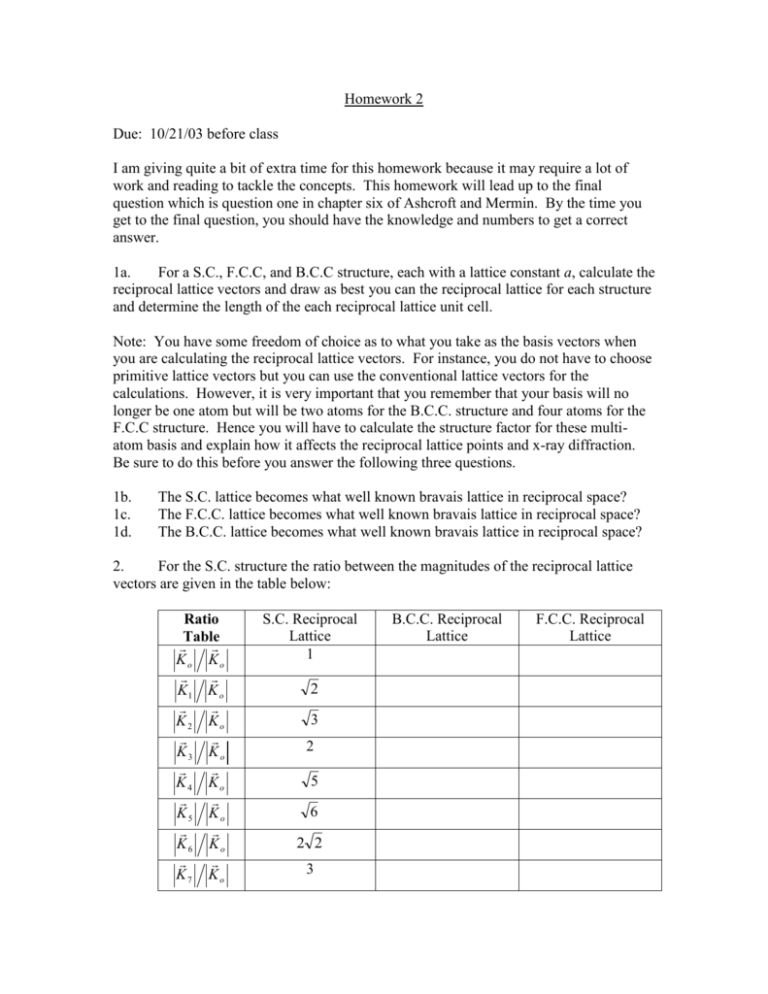
Homework 2 Due: 10/21/03 before class I am giving quite a bit of extra time for this homework because it may require a lot of work and reading to tackle the concepts. This homework will lead up to the final question which is question one in chapter six of Ashcroft and Mermin. By the time you get to the final question, you should have the knowledge and numbers to get a correct answer. 1a. For a S.C., F.C.C, and B.C.C structure, each with a lattice constant a, calculate the reciprocal lattice vectors and draw as best you can the reciprocal lattice for each structure and determine the length of the each reciprocal lattice unit cell. Note: You have some freedom of choice as to what you take as the basis vectors when you are calculating the reciprocal lattice vectors. For instance, you do not have to choose primitive lattice vectors but you can use the conventional lattice vectors for the calculations. However, it is very important that you remember that your basis will no longer be one atom but will be two atoms for the B.C.C. structure and four atoms for the F.C.C structure. Hence you will have to calculate the structure factor for these multiatom basis and explain how it affects the reciprocal lattice points and x-ray diffraction. Be sure to do this before you answer the following three questions. 1b. 1c. 1d. The S.C. lattice becomes what well known bravais lattice in reciprocal space? The F.C.C. lattice becomes what well known bravais lattice in reciprocal space? The B.C.C. lattice becomes what well known bravais lattice in reciprocal space? 2. For the S.C. structure the ratio between the magnitudes of the reciprocal lattice vectors are given in the table below: Ratio Table Ko Ko K1 K o K2 Ko K3 Ko K4 Ko K5 Ko K6 Ko K7 Ko S.C. Reciprocal Lattice 1 2 3 2 5 6 2 2 3 B.C.C. Reciprocal Lattice F.C.C. Reciprocal Lattice Now I want you to calculate similar data for the F.C.C. and B.C.C. structures. Calculate the eight smallest ratios (the ratio is defined to be with respect to the reciprocal lattice vector with the smallest magnitude for that particular reciprocal lattice) of the reciprocal lattice vectors for the F.C.C. and B.C.C. reciprocal lattice points. Remember that two reciprocal lattice vectors that have the same magnitude only count as one! If you used multi-atom basis to calculate the reciprocal lattices, be sure to disregard any reciprocal lattice point that has a structure factor of zero. (Show your work) 3. As I have said before, the diamond lattice can be viewed as either two interpenetrating F.C.C. lattices or also as an F.C.C. lattice with a basis of two atoms. Use this second view to help you in calculating the structure factor for a diamond lattice. Indicate how it affects the x-ray intensity peaks of the standard F.C.C. structure. Note: Again, there are several ways to do this problem. One way that may be easier than other ways is to consider the diamond structure as a simple cubic crystal structure with a basis of eight atoms. You may find the reference in the following webpage useful: http://www.stoner.leeds.ac.uk/teaching/CM2/CM6/sld001.htm 4. Now armed with solutions to problems 1-3, onto the problem in Ashcroft and Mermin. Identify the F.C.C. and B.C.C. structure and calculate the lattice constant. (Extra credit) Identify the diamond structure and calculate the lattice constant. You may find the formula for the magnitude of the reciprocal lattice vector useful: K 2k sin 2 Hint: Compute the magnitudes of K, divide these values by the magnitude of the K that has the smallest magnitude. This will get you the ratios which should match your results for problem 2.