Density-form-2008
advertisement

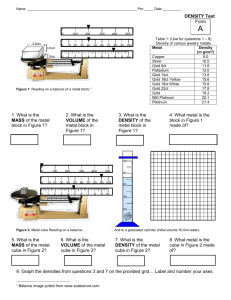
Name(s): _______________ Chem 120 / Dr. Rusay DENSITY & Measurement Complete the following: Unknown Number Reference # ___________ Measure and record the cylinder's length. ___________ Measure and record the cylinder's diameter. ___________ Weigh and record the mass of the cylinder. ___________ DENSITY of cylinder (g/cm3) ___________ Identity of metal ___________ Volume of water in graduated cylinder + metal cylinder. ___________ Volume of water in graduated cylinder ___________ Volume of metal cylinder. ___________ Which method is most accurate in determining the density of the metal cylinder? Briefly explain your choice. Consider the Purdue University students’ data for the density of a U.S. penny. Rank the four methods in order of increasing Precision & Accuracy: Precision: ___ < ___ < ___ < ___ Briefly explain your choices. Accuracy: ___ < ___ < ___ < ___ Experimentally Determining Density Significant Figures, Accuracy, Precision and Data Analysis Complete the form on the opposite side of this page: one per group with each group member’s name is to be turned-in when finished. 1. Calculate the density of a metal cylinder using your linear measurements and the mass. Be sure to have the correct number of significant figures. Identify the metal from its density and show your results to Dr. R. 2. Using the same cylinder or a cylinder made of the same metal, record it’s mass and measure its volume by displacement using a graduated cylinder and water. Enter your data on the form. Calculate the density. Record the value and provide a brief discussion of which method is more accurate. A group of students at Purdue University applied these methods to “new” and “old“ pennies. (NOTE: Both the “new” and “old“ pennies that they used in the experiment were in public circulation and not in mint condition.) A group of students followed the following four different procedures for one set of “new” pennies and another group repeated the measurements with “old” pennies. Method 1: The mass, height and diameter of a stack of ten pennies were measured respectively using an analytical balance and calipers. The student data is provided in Table 1. Method 2: The mass, and volume of a stack of ten pennies were measured respectively using an analytical balance and 100 mL graduated cylinder. The student data is in Table 2. Method 3: The mass, height and diameter of a single penny were measured respectively using an analytical balance and calipers. The student data is provided in Table 3. Method 4: The masss, and volume of a single penny were measured respectively using an analytical balance and 10 mL graduated cylinder. The student data is in Table 4. However, unlike diligent and meticulous DVC Chem 120 students, the Purdue students failed to record whether the data they obtained was for “new” or “old“ pennies. 3. Your indivdual challenge is to determine in your breakout subgroup which of the data sets that you’ve been assigned to analyze is for the “new” and which is “old“ and the precision and accuracy of the method. You must use significant figures and knowledge of mass and volume measurements correctly. 4. Returning to your parent group, select one of the four methods as being the best experimental approach in obtaining the most precise and accurate results, and then ranking the four methods respectively in increasing order of 1) precision and 2) accuracy.