Porphyrin Ring Synthesis and Breakdown
advertisement

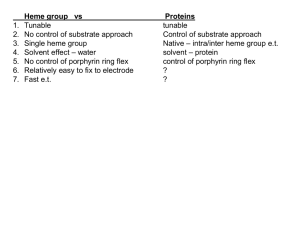
Porphyrin Ring Synthesis and Breakdown Daniel Wellman What are they? • Porphyrin rings are biological molecules used in a variety of essential chemical processes •The two most well-known porphyrins are heme and chlorophyll Heme Because of their large conjugated double bond system, porphyrins typically absorb visible light Chlorophyll’s green color, heme’s red, and the blue blood of some sea creatures are all a result of this absorbance Additionally, the 4 nitrogen atoms at the center of the ring are excellent at conjugating metals because of their lone pairs As a result, porphyrins are a common way to attach metals to proteins • Porphyrin synthesis is still poorly understood • Intermediates are known, but reaction mechanisms are generally still speculative • Plants and animals follow different synthetic pathways in the early stages of ring formation • Once delta-aminolevulinate is synthesized, the synthetic pathways are identical • Delta-aminolevulinate is used only for porphyrin synthesis, so its synthesis is tightly regulated— at least 6 enzymes or products are controlled Beginning steps in plants Glutamate glutamyl-tRNA O ATP->AMP O NH3 NH3 O O O NADPH O t RNA-Glu O glutamate 1-semialdehyde delta-aminolevulinate O O NH3 O O H O NH3 O glutamyl-tRNA glutamate 1-semialdehyde O O NH 3 O GluANRt O NADPH NH3 O H O This reaction uses Glu tRNA as a leaving group, which is very unusual Guu tRNA acts like a phosphate group, facilitating transformation of a carboxylic acid to an aldehyde Because of the size of the tRNA molecule, the enzyme must be highly flexible The only well-understood mechanism is the last step Structure of glutamate-1-semialdehyde 2,1aminomutase To limit access to the highly reactive PLP active site, the enzyme uses a “gating loop,” an alpha-helix that changes into a beta-sheet to expose the active site of the enzyme This prevents a variety of side reactions from happening What about people? Glycine Succinyl-CoA O O + S-CoA NH3 O O O O delta-aminolevulinate O O O O NH3 O O NH3 O • For animals and some eukaryotes, synthesis of delta-aminolevulinate follows a different path What’s Next? The next step is the synthesis of Porphobilinogen from 2 molecules of delta-aminolevulinate delta-aminolevulinate O O O NH3 O O O O O O N NH3 O NH3 H Proposed mechanism for Porphobilinogen synthesis • Another cool thing about this enzyme is that it contains zinc, but the zinc does not react in any way • Experiments determined that zinc is used to control reactivity and make the catalytic site more selective This reaction mechanism is fairly complicated, but isotope experiments have confirmed which atoms come from which precursor molecules The results of these studies are shown in blue and red O O O O O O O O O O O O 4 HN NH HO HN NH N NH3 H O O O O O O O O Ring closure O O O O O O O O O O O O O O H O O HN NH HN NH H HN NH O HN NH O O O O O O O O O O O O O Mechanism for ring closure Steps after ring formation Essentially all that is needed after these steps is insertion of the metal at the center of the ring Additional covalent modification of the outside of the ring is often performed Examples of this modification include siroheme and chlorophyll Heme Iron Insertion • Metal insertion in porphyrins is performed by a class of complexes called chelatases Ferrochelatase Ferrochelatase activity To force iron (II) into the heme ring, ferrochelatase holds the ring in a bent conformation This bending causes the ring to pop out of the enzyme once iron insertion is complete What do you do with it once you’re done with it? • Recycling the iron in heme is critical for survival • To make sure iron is not unnecessarily wasted, a complex system for recycling the iron in heme is employed • The ring itself is solubilized and eliminated—only the metal is reused Heme Breakdown Followed By… • Reaction with 2 molecules of sugar to yield: Bilirubin diglucuronide So what happens if you have a problem making or breaking down porphyrins? A variety of medical conditions can occur Babies often suffer from jaundice, which is caused by an accumulation of heme breakdown products in fat Jaundice is typically harmless, and typically is cured with exposure to light The condition fades once proper liver function develops in the infant There are some serious conditions that result from accumulation of porphyrins The most severe of these is porphyria, which is caused by a problem in one of the first 8 steps of synthesis Porphyria • Porphyria is assumed to be the root of our myths about vampires and werewolves • In all cases, the urine changes colors, typically to red, but occasionally to pink or purple, and the teeth become reddish-brown, and the lack of heme means that sufferers are anemic • In erythroid porphyrias, the skin becomes extremely sensitive to light, and will blister or scar if exposed, and hair will grow in unusual places • Ulcers may also cause their hands to become deformed and pawlike and mutilate the nose, ears, eyelids, and fingers • In hepatic porphyrias, damage to the central nervous system results, often leading to seizures or insanity • Because of the wide variety of symptoms, porphyria has been associated with insane rulers like King George III