Chapter 3 Atomic vibration and phonons
advertisement

Chapter 3 Atomic Vibration and Phonons
1. Monatomic Linear Chain
0
a
2a
3a
u1
u2
u3
u4
x
(N2)a (N1)a
uN1
uN
A linear atomic chain contains N atoms, each with mass
m, at x = 0, a, 2a, 3a …. (N1)a.
The displacement of the nth atom from its equilibrium
position is un.
Considering nearest neighbor interaction only, the
equation of motion of the nth atom is (except those near
the boundary) :
2u
d
n = ( un+1 un) ( un un1),
m
(1)
2
dt
where is the force constant.
The number of equation is N ( 1023). The atoms near the
edges would have an environment different from that at
the interior. The equations of motion of these atoms
would be different from (1). However, these atoms have
a small population, they are assumed to obey Eq.(1)
without affecting the results. This assumption can be
realized by assuming that the original atomic chain is part
of an infinitely long chain.
1
Periodic boundary condition
The original crystal has N equations of motion for each
atom. The extended imaginary crystal has more equations.
We keep the number of equations to be N by introducing
the periodic boundary condition:
un(t) = un+N(t)
The motion of an atom in the crystal is assumed to be
exactly identical to another atom at the corresponding
position of the added imaginary crystal.
(nN)th
nth
(n+N)th
……. …. …. …. ……..
imaginary
real
imaginary
2
To solve the equations of motion, use the trial solution :
un(t) = A exp i(qna t), where A is the amplitude, q =
2/ = propagation constant and = angular velocity.
Equation (1) becomes :
m 2 A exp i(qna t)
= [Aexp i(q(n+1)at) Aexp i(qnat)]
[Aexp i(qnat) Aexp i(q(n1)at)]
m 2 = [exp (iqa) + exp (iqa) 2]
m 2 = 2 (1 cos qa)
Dispersion relationship correlates q and
(one q corresponds to one for a linear chain).
(q) = (4/m)1/2 sin qa/2 .
> 0,
max = 2(/m)1/2
max = 2(/m)1/2
q
-3/a
-2/a -/a
0
3
/a
2/a
3/a
(q) is periodic, because
(q+2/a) = (4/m)1/2 sin (q 2/a)a
2
= (4/m)1/2 sin (qa/2+ )
= (q)
Period is 2/a.
Allowed values of q's are discrete because:
un(t) = un+N(t),
exp i(qnat) = exp i[q(N+n)at),
1 = exp i qNa,
qNa = 2l, where l is an integer
2l
, l = …-2, -1, 0, 1, 2, …
Na
2
2. q's are equally spaced by q =
.
Na
1. Allowed q =
An allowed q would give an . A q value defines a
collective motion of the atoms, which defined as a
normal mode of the crystal vibration.
Total number of allowed q values is fixed, because
un,q+2/a(t) = A exp [i(q+2/a)na (q+2/a)t]
= A exp i(qna (q)t) (for (q) is periodic)
= un,q(t).
Conclusion: The normal modes described by q and
q+2/a are identical. The period of q is 2/a.
4
The range [/a, /a] is selected to define the First
Brillouin zone to include all the allowed q values.
Reciprocal lattice vector A = 2/a x̂
1st Brillouin zone : [/a, /a]
1st B.Z.
2/a
/a
0
A
G
/a
x̂
2/a
2
2 2
)/q = ( )/( ) = N
a
a
Na
= no. of atoms = no. of primitive unit cells
The number of allowed q is (
Long wavelength limit refers to the modes with small q
very close to 0 (or equivalently ). Hence,
qa
)1/ 2 q.
( 4m )1 / 2
= a (m
2
Sound speed is defined at the long wavelength limit
)1/ 2
v = = a ( m
q
5
Example : Prove that the modes described by q ( = 3.3a)
and q+2/a (' = 0.76a) are equivalent.
Note: The physical meaning of this result is that
atoms in a solid are discrete particles,
while the material is not a continuous
medium.
u
6
2.Diatomic linear chain :
A diatomic linear crystal is a 2-D crystal consisting of two
kinds of atoms.
¤
M
m
a/2
M
¤
M
m
¤
m
x
a/2
Equations of motion for M and m in the nth cell are:
d2 uM n (t )
M
= ( um n uM n) ( uM n um n1)
(2)
d t2
2
m d um 2n (t) = ( uM n+1 um n) ( um n uM n)
(3)
dt
Apply the trial solution:
uM n(t) = AM exp i(qna t), and
um n(t) = Am exp i[qa(n+1/2) t].
(4)
(5)
M2 AM = 2[ Am cos( qa ) AM], and
2
m2 Am = 2[ AM cos( qa ) Am].
2
(6)
(2 M2) AM 2 cos( qa ) Am = 0, and
2
qa
2 cos( ) AM + (2 m2) Am = 0.
2
(8)
7
(7)
(9)
Dispersion relationship
If Eq.(8) and (9) have a non-zero solution, then:
2 M 2
2cos (qa / 2)
= 0.
2
2cos (qa / 2)
2 m
By solving the equation, the dispersion relationship is :
2 = M m [( M m )2 4 sin2(qa/2)]1/2
(10)
Mm
Mm
Mm
Different from a monatomic chain, one q gives two 's.
has two branches :
"+" for Optical branch
"" for Acoustic branch
optical branch
acoustic branch
-/a
0
8
/a
q
Example 1
Optical branch at q =/a, has the minimum value of :
2 = M m [( M m )2 4 sin2(qa/2)]1/2
Mm
Mm
Mm
(10)
op, min = { M m + [( M m )2 4 ]1/2}1/2
Mm
Mm
Mm
2 2Mm m2
M
M
m
4 ]1/2}1/2
= {
+[
Mm
Mm
(Mm)2
= [ M m + M m ]1/2 = (2/m)1/2
Mm
Mm
9
Example 2
Optical branch at q = 0, has the maximum value :
2 = M m [( M m )2 4 sin2(qa/2)]1/2
Mm
Mm
Mm
(10)
op, max = { M m + [( M m )2 0]1/2}1/2
Mm
Mm
= (2 M m )1/2
Mm
Band width = op, max op, min = (2 M m )1/2 (2/m)1/2
Mm
10
From (8) or (9)
(2 M2) AM 2 cos( qa ) Am = 0
2
(8)
for the optical mode at q = 0 :
(2 M op, max 2) AM 2 cos 0 Am = 0
(2 M op, max 2) AM 2 Am
=0
AM
2
=
=
=m <0
M
m
Am
2 2M M m Mm
Mm
AM M + Amm = 0 = (M + m) AC.G.
Since the C.G. of the unit cell does not move. An optical
mode describes the relative motion of atoms within a
basis.
An optical mode can be excited by EM wave.
The mode is termed "optical" since it can be excited by
an EM wave.
positive charge
negative charge
11
Example 3
2 = M m [( M m )2 4 sin2(qa/2)]1/2
Mm
Mm
Mm
(10)
Acoustic branch at q 0, has the minimum value :
ac, min2 M m M m [1 4Mm 2 (qa/2)2]1/2
Mm
Mm
(M m)
M m M m [1 1 Mm 2 (qa)2]
Mm
Mm
2 (M m)
2a2
q
=
2(M m)
Amplitude of atoms, from (8): (2 M2) AM2cos(qa/2) Am = 0
(2 Mac, min 2) AM 2 Am = 0
AM
2
= 2/(2 Mac min2) =
1
2
2
Am
2 M q a
2M m
AM Am, amplitudes of vibration of the two kinds of
atoms in the same unit cell are about the same. The
motions of the atoms are approximately in phase.
q 0 is the condition of long wavelength limit,
2
2
ac, min ( a
)1/2q, sound speed = ( a
)1/2
2(M m)
2(M m)
+ charge
- charge
12
Show that is periodic. Since
2(q) = M m [( M m )2 4 sin2(qa/2)]1/2
Mm
Mm
Mm
2
(q+2/a) = …. {…. … sin2[(q+2/a)a/2]}1/2
= …. {…. … sin2 (qa/2)}1/2
= 2(q)
(q) has a period of 2/a.
Allowed values of q are discrete, since periodic boundary
condition ensures :
uM n = uM n+N
AM exp i(qna t) = AM exp i(q[n+N]a t)
qNa = 2h
allowed q of a normal mode has the form of
q = 2h/(Na) , where h is an integer.
q = 2/Na
Total no. of allowed q is limited, since :
uM n(q+2/a) = AM exp i( [q+2/a] na (q+2/a) t)
= AM exp i(qna (q) t)
= uM n(q)
Therefore uM n(q+2/a) and uM n(q) represent the same
normal mode.
Define the range [/a, /a] to be the 1st Brillouin zone.
2 2
No. of allowed q = ( )/( ) = N = no. of unit cells
a
Na
13
No. of normal modes = (no. of q's) x (no. of branches) =
no. of allowed = 2N.
Note : In general, the no. of normal modes of a crystal = DnN,
D = the dimension of a crystal (i.e. 3 for 3-D crystal, 2 for
planar, 3 for chain)
Dn = no. of branches
= no. of acoustical branches + no. of optical branches
= D + D(n 1)
N = no. of primitive unit cells
= no. of allowed q
Example : Dispersion relationship of diamond.
D = 3, n = 2 (primitive unit cell)
Three acoustic branches, three optical branches.
No. of normal modes = 6N
LO
LO
TO
LA
TO
LA
TA
TA
q
14
3. Vibration of 3-D crystal
q vectors are discrete, since from the periodic boundary
condition
:
u q( Rn , t) = u q( Rn +N1 a + N2 b + N3 c , t),
where Rn is the lattice vector of the nth atom, N1, N2 and
N3 are integers.
z
N2b
N3c
N1a
c
x
y
a
b
Aqexp i( q Rn t)= Aqexp i( q [ Rn +N1 a +N2 b +N3 c ]t),
q N1 a = 2n1
q N2 b = 2n2, and
q N3 c = 2n3,
where n1, n2, n3 are integers.
n1 n2 n3
C.
The solution of q is : q =
(11)
A+
B+
N3
N1
N2
n1 n2 n3
A+
B+
Proof : N1 q a = N1 (
C) a
N1
N3
N2
= N1 (n1/N1) 2 + 0 + 0
= 2 n1
15
No. of q vectors are finite. If
q = (n1/N1) A + (n2/N2) B + (n3/N3) C and
q ' = [(n1+ N1)/N1] A + (n2/N2) B + (n3/N3) C
= (n1/N1 + 1) A + (n2/N2) B + (n3/N3) C
are two allowed wave
vectors.
uq = Aqexp i( q Rn t).
u = Aqexp i( q ' Rn t)
q'
=Aqexp i{[(n1/N1+1) A +(n2/N2) B +(n3/N3) C ] Rn t}
= Aqexp i( q Rn + A Rn t)
(Note : Rn = m a + n b + p c )
= Aqexp i ( q Rn + m A a t)
= Aqexp i ( q Rn + 2m t), and
= Aqexp i( q Rn t)
= u q( Rn , t)
Both q and q ' index the same normal mode.
The first Brillouin zone is :
[ A /2, A /2], [ B /2, B /2] and [ C /2, C /2]
16
Example : The first Brillouin zone of a 2-D crystal.
Exercise : With the aid of the diagram, show that for a
vector q in reciprocal lattice space, from the
zone center
to the zone boundary is
q G G 2/2 = 0.
G
q
Examples : 1st B.Z. of S.C.
B.C.C. (reciprocal space is F.C.C.)
k
P
H
i
17
N
j
4. Number density of normal modes in a reciprocal lattice
space
st
q 's are equally spaced in the 1 B.Z. bounded by A /N1,
B /N2 and C /N3. All the q ’s are in the 1st Brillouin
zone having a volume of:
A ( B C )/(N1N2N3)
2
= ( b c ) ( B C )/(N1N2N3)
2
= [( b B )( c C ) ( b C )( c B )]/(N1N2N3)
= 2 [( b 2c a )( c 2a b ) 0]/(N1N2N3)
= (2)3/(N1N2N3)
= (2)3/Vc
(Vc volume of the specimen)
Number density of q in a reciprocal space
= number density of normal modes = 1/[23/Vc] = Vc/(2)3.
18
5. Density of modes
At the long wavelength limit ( = vq) of an acoustic
branch, a sphere in the reciprocal space with a radius q
cuts some allowed q ’s. They represent the normal modes
having the frequency .
The number of modes with frequencies between and
+d correspond to all the q ’s lying between the two
spheres.
qy
Reciprocal lattice
space
qx
Density of normal modes g()
g() d = no. of modes between and +d
Vc
V
= Volume between
dq3 = c 4q2 dq
the spheres with
(2)3
(2)3
=
radii and d
2
Vc
4 2 d(
)
3
(2)
Vc2
Density of normal modes = g() = 2 3
2
19
For a 3-D crystal, there are three acoustic branches, so
that
V 3 1
g() = c2 (
) 2
2 s 1 3
s
Density of states of Copper
0
1
2
3
4
(1013 rad s-1)
20
5
6. Debye Approximation
Since the number of acoustic modes is 3N (a finite number),
there is a maximum denoted as D, such that :
D
3N =
g() d
0
D
Vc 3 1
2
d
(
)
22
0
s 1 s 3
VcD 3 1
=
(
)
2
3
6 s 1
s
=
2
Debye frequency D = [
3
1/ s 3
]1/3
s 1
S = 1, 2, 3 for the three acoustic branches.
Define Debye Temperature TD = D/kB,
kB is the Boltzmann's constant = 1.38 10-23 J K-1
21
7. Phonons
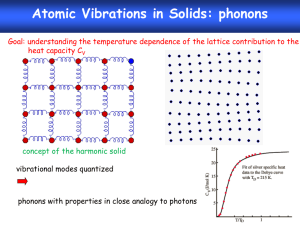
The energy of a simple harmonic oscillator is quantized
in the form of E = (n + 1/2), where n = 0, 1, 2, …
The total energy of a normal mode is Ei,n= i(n+1/2).
An energy quantum i is defined as a phonon. It is the
energy quanta associated with a collective motion of all
the atoms in one vibration mode.
A normal mode has equally spaced energy levels.
3(1+1/2)
2(2+1/2)
1(4+1/2)
1(3+1/2)
2(1+1/2)
1(2+1/2)
3/2
1(1+1/2)
2/2
E = 1/2
Mode q1
q2
………..
qn
A set of integers {n1, n2, n3, ….} is used to describe the
overall vibration status of a solid.
22
8. Average phonon numbers in a mode is defined as <n>, so
that the average vibration energy is <E> = (n +1/2).
(n 1/ 2) exp(-[n 1/ 2] / kBT )
<E> = n 0
exp(-[n 1/ 2] / k T )
B
n0
= n exp(nx)
(set x = /(kBT) )
exp(nx)
= d ln exp(nx)]
dx
1
= d ln
]
dx 1 exp(x)
= d ln[1 exp( x)]
dx
=
=
exp (kBT ) 1
exp(x) 1
<E> = (
+ 1/2)
exp (kBT ) 1
Comparison with E = (n + 1/2), one defines the
average phonon no. <n> =
exp (kBT ) 1
Exercise : Show that at high temperatures << kBT,
(i) <n> kBT/( )
(ii) <E> kBT (the classical result)
Exercise : Show that at low temperatures >> kBT,
(i) <n> exp( /kBT)
(ii) <E> exp( /kBT) + /2
23
11. Debye model of heat capacity
g (d + g (d
<E>all atoms=
exp (kBT ) 1
0
0
High temperatures, << kBT,
exp( /kBT) 1 1+ /(kBT)1 = /(kBT)
g (d + g (d
<E>all atoms
kBT
0
0
Cv = 3 NkBT = constant (classical)
Low temperatures, >> kBT,
2
1
Using TD =
[
] 1/3
kB
1/ s 3
V
1
2
g() = c2
2 1/ 3
s
3 2
VcN
3
2
9
N
1/
= 9N(
s ) =
kB3TD3
182
4
<E> = 9N
kB3TD3
D
0
d +
exp (kBT ) 1
0
g (d
if x = /(kBT) >> 1, xmax = D(kBT) .
x
dx + g (d
<E>all atoms = 9NkBT4/TD3
exp x 1
0
0
24
= 9NkBT4/TD3 (4/15) +
4
4
3
= 3 NkBT /5TD +
g (d
0
g (d
0
Cv
= 124NkBT3/5TD3 T3.
Heat capacity
Pb
Al
Si
Diamond
0
400
T (K)
25
800
9. Thermal conductivity
Consider the contribution of phonons only.
Two regions in the solid are separated by a mean free
path of a phonon l (distance that a phonon can travel
without being scattered)
The net energy flux passing through the interface is Je =
nv<E>1 nv<E>2. n is the phonon density. <E>1 and
<E>2 are the phonon energies at points A and B. v is the
drift velocity of a phonon.
Energy current density is
Je = nv (<E>1 <E>2) = nv ( dT l ) c, where c is the
dx
specific heat capacity of a normal mode.
is the thermal conductivity, where Je = dT . Therefore,
dx
nclv or Clv, where C = specific heat per unit volume.
Interface
l
A
B
T
T+ dT l
dx
26