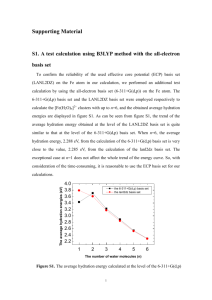
Theoretical studies on the properties of uracil and its dimer upon t
advertisement

Theoretical studies on the properties of uracil and its dimer upon thioketo substitution Weihua Wanga, Nana Wanga, Ping Li*,a,b, Yuxiang Bu*,a,b, Xiaoyan Xiea, Rui Songa a b College of Chemistry Science, Qufu Normal University, Qufu, 273165, P. R. China Key Laboratory of Colloid and Interface Chemistry (Shandong University), Ministry of Education, Jinan, 250100, P. R. China Supporting Information Evaluation of the reliability of the computational level As mentioned in the paper, the DFT (B3LYP) method in conjunction with the 6-311+G* basis set has been employed throughout the calculations. Generally, inclusion of electron correlation provided by the DFT method enables one to avoid using the more computationally expensive post-Hartree-Fock methods. As for the present study, it is difficult to perform the high level calculations like MP2 or CCSD levels to our best computational ability due to the large size of the systems (up to 466 basis functions in some cases). However, some pioneering studies including experiments and high level theories can be compared to assess the reliability of the computational level used here. The main comparisons are summarized as follows: (1) As is well known, in most cases, DFT method (e.g., B3LYP) can provide well-consistent results with those at the MP2 level as for the relevant DNA base pairs or fragments [1-5]. On the other hand, for the systems in which dispersion energy plays a dominant role, such as stacked DNA base pairs, DFT fails to locate the corresponding stacked structures due to the lack of London dispersion term [6-7]. Fortunately, in our study, none of the stacked structures exists and all the dimers are characterized by the intermolecular H-bonds. As expected, the calculated interaction energies at B3LYP level are slightly lower relative to those at MP2 or CCSD levels. For the H-bonded nucleic acid base pairs, it is reported that B3LYP underestimates the interaction energies by few kcal/mol with relative error of 2.2 kcal/mol [1]. For the most stable uracil dimer, the calculated pairing energy of 15.5 kcal/mol at the B3LYP/6-311+G* level of theory is well consistent with the result of 15.9 kcal/mol at the MP2/6-31G*(0.25)//HF/6-31G** level of theory [8]. Here, the benchmark value is 19.04 kcal/mol at the CCSD(T) level [9]. Despite the slight underestimation of the pairing energy at the B3LYP level, the change tendency for the pairing energy of uracil dimer upon thioketo substitution can be predicted correctly since they are calculated at the same computational level. At the same time, the reliability of B3LYP method in treating the relevant H-bonded uracil systems has been confirmed previously through comparison of the results using the MP2 method [3-5]. Thus, the calculated results for the changes of geometry and pairing energy should be reliable at the B3LYP/6-311+G* level of theory. (2) The reliability of the B3LYP/6-311+G* level of theory can be also reflected from the calculated dipole moment of uracil. As shown in Table S1, the calculated dipole moment in the present study is well consistent with the results available theoretically and experimentally [9,10-16]. For example, the present 4.61 D lies in the range of previous theoretical results varying from 4.1 to 5.28 D, which is slightly larger than those of experimental results (3.86 and 4.16 D) [15-16]. At this point, it has been reported that MP2 method gives a slightly larger dipole moment than B3LYP at the 6-31+G** basis set (5.28 versus 4.66 D) and the calculated dipole moments are quite sensitive to the basis set used [10]. Moreover, the dipole moment can be further reduced if larger basis sets are employed, where a better value (4.33 D) has been reported at the RIMP2/AUG-cc-pVTZ level of theory [9]. Similarly, the result of the dipole moment can be also improved for B3LYP method employing larger basis sets. Actually, the calculated results are 4.58 and 4.48 D at the B3LYP/6-311++G** and B3LYP/AUG-cc-pVTZ levels of theory, respectively. (3) The ionization potentials (IPs) including vertical and adiabatic ones can be used to evaluate the reliability of the B3LYP/6-311+G* level of theory. As shown in Table S2, at larger basis set 6-311++G(3df,2p), there is not clear improvement on the calculated results. Most importantly, the calculated ionization potentials are well consistent with the available experimental data [18], further confirming the reliability of the level of theory employed. (4) At the B3LYP/6-311+G* level of theory, the calculated vertical and adiabatic singlet-triplet energy gaps of 3.64 and 2.99 eV are well consistent with the results (3.62 versus 3.00 eV) at the B3LYP/AUG-cc-pVTZ level of theory [17]. Most importantly, both of the vertical results are in good agreement with the experimental value of 3.65 eV [19], For the adiabatic energy gap, both DFT and CCSD(T) methods yield similar values within 0.1 eV [17]. Especially, Nguyen et al noted that the second-order perturbation theory (U)MP2 consistently yields too large energy gaps and could thus not be used as a predictive method [17]. Thus, in this sense, it is better to choose the B3LYP instead of MP2 method to investigate the single-triplet energy gaps of uracil and its dimer upon thioketo substitution. (5) Additionally, the reliability of the B3LYP/6-311+G* level of theory can be also reflected from the calculated gas phase acidities. As shown in Table S3, the calculated results for uracil are well consistent with the available experimental and theoretical results including B3LYP and MP2 methods [5,20-22]. On the other hand, the MP2 method appears to underestimate the acidity at the N3 site compared with the experiments. For the thioketo substituted cases, the relative acidity between the N1 and N3 sites in each monomer is also consistent with the previous results as well as the relative acidities among the different monomers. Overall, as described above, the reliability of the B3LYP/6-311+G* level of theory should be confirmed through the comparison of the results with the available experimental results and high level calculations. Table S1 The calculated dipole moment of uracil (Debye) together with the available experimental and theoretical results Present* 4.61/4.58/4.48 Ref.[11] Ref.[12] 4.1 4.21 Ref.[9] 4.33 Ref.[13] Ref.[14] Ref.[10] Ref.[15] Ref.[16] 4.4 5.11 5.28 3.86 4.16 * Obtained using the B3LYP method at the 6-311+G*, 6-311++G**, and AUG-cc-pVTZ basis sets, respectively. Table S2 The calculated vertical and adiabatic IPs (eV) together with the available experimental and theoretical results VIP 9.49a 9.46b 9.45c 9.50±0.03c AIP 9.27a 9.22b 9.20c 9.4±0.1c a In the present study. At the B3LYP/6-311++G(3df,2p) level of theory in Ref.[17]. c Ref.[18]. b Table S3 The calculated gas phase acidity of uracil and its thioketo substituted species together with the available experimental and theoretical results* Complexes Present Ref.[5]a Ref.[20]b Ref.[21]c U 330.0/343.3 332.5/345.9 329.1/340.9 333±4/347±4 2SU 323.2/335.5 326.1/334.8 4SU 322.1/336.4 325.0/339.0 24SU 317.0/330.0 320.0/332.8 * The data before and after slash refer to the acidity of N1 and N3 site, respectively. a At the B3LYP/6-31+G(d,p) level of theory. b At the MP2(full)/6-311+G(2d,2p)//MP2(full)/6-31G* level of theory. c Experimental results. Ref.[22]c 333±5 References [1] J. Šponer, P. Jurečka, P. Hobza, J. Am. Chem. Soc. 2004, 126, 10142. [2] A. Hocquet, N. Leulliot, M. Ghomi, J. Phys. Chem. B 2000, 104, 4560. [3] A. K. Chandra, M. T. Nguyen, T. Uchimaru, T. Zeegers-Huyskens, J. Phys. Chem. A 1999, 103, 8853. [4] M. T. Nguyen, A. K. Chandra, T. Zeegers-Huyskens, J. Chem. Soc., Faraday Trans. 1998, 94, 1277. [5] E. Kryachko, M. T. Nguyen, T. Zeegers-Huyskens, J. Phys. Chem. A 2001, 105, 3379. [6] I. Dąbkowska, P. Jurečka, P. Hobza, J. Chem. Phys. 2005, 122, 204322. [7] J. Šponer, J. Leszczynski, P. Hobza, J. Phys. Chem. 1996, 100, 1965. [8] M. Kratochvíl, O. Engkvist, J. Šponer, P. Jungwirth, P. Hobza J. Phys. Chem. A 1998, 102, 6921. [9] J. A. Frey, A. Mu1ller, M. Losada, S. Leutwyler, J. Phys. Chem. B 2007, 111, 3534. [10] A. F. Jalbout, B. Trzaskowski, Y. Xia, Y. Li, X. Hu, H. Li, A. El-Nahas, L. Adamowicz, Chem. Phys. 2007, 332, 152. [11] R. E. A. Kelly, L. N. Kantorovich, J. Phys. Chem. B 2006, 110, 2249. [12] J. Leszczyiiski, J. Phys. Chem. 1992, 96, 1649. [13] J. Šponer, J. Leszczynski, P. Hobza, Biopolymers 2001, 61, 3. [14] M. T. Rodgers, P. B. Armentrout, J. Am. Chem. Soc. 2000, 122, 8548. [15] R. B. Brown, P. D. Godfrey, D. McNaughton, A. P. Pierlot, J. Am. Chem. Soc. 1988, 110, 2329. [16] I. Kulakowska, M. Geller, B. Lesyng, K. L. Wierzchowski, Biochim. Biophys. Acta 1974, 361, 119. [17] M. T. Nguyen, R. Zhang, P. Nam, A. Ceulemans, J. Phys. Chem. A 2004, 108, 6554. [18] Values taken from the NIST standard reference database: http://webbook.nist.gov/. [19] R. Abouaf, J. Pommier, H. Dunet, P. Quan, P. Nam, M. T. Nguyend, J. Chem. Phys. 2004, 121, 11668. [20] Z. Yang, M. T. Rodgers, J. Phys. Chem. A 2006, 110, 1455. [21] M. A. Kurinovich, J. K. Lee, J. Am. Chem. Soc. 2000, 122, 6258. [22] T. M. Miller, S. T. Arnold, A. A. Viggiano, A. E. S. Miller, J. Phys. Chem. A 2004, 108, 3439. (a) (b) (c) (d) (e) (f) Figure S1. The total energy changes along with optimization step number for the designed possible stacked uracil dimmers, where the selected geometries in the optimization process have also been displayed. Figure S2. The total energy changes along with optimization step number for the designed stacked structure of uracil with the Zn2+ on the top of the ring, where the selected geometries in the optimization process have also been displayed. Figure S3. The scanned potential energy curve for the stacked structure with the Zn2+ on the top of the uracil ring versus the distance of R. (a) (b) (c) (d) (e) (f) Figure S4. The total energy changes along with optimization step number for the designed possible sandwiched structures with Zn2+ ion between two uracil rings, where the selected geometries in the optimization process have also been displayed.