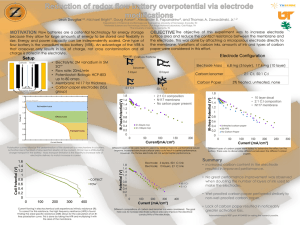
Calibration of pH meters and glass electrodes at the National
advertisement

Calibration of pH meters and glass electrodes at the National Physical Laboratory of Israel Elena Kardash and Ilya Kuselman The National Physical Laboratory of Israel (INPL), Givat Ram, Jerusalem 91904, Israel, tel.: +972-2-6536-534; fax: +972-2-6520-797; e-mail: lena.ka@moital.gov.il pH is one of the most frequently measured physico-chemical properties in fields of health care, safety, pharmaceutical industry, environmental control and others [1]. The pH value is defined as the negative decimal logarithm of the hydrogen ion activity aH: pH = -lg aH (1) Only in highly dilute (so-called “ideal”) solutions, the activity is equal to the hydrogen ion concentration. Single ion activity is not measurable by any thermodynamically valid method and requires a convention for its evaluation. For this reason, a pH scale is based on the pH values of a series of standard buffer solutions determined by the potentiometric technique [2]. Routine potentiometric measurements of pH are carried out by means of a pH meter, accompanied by a temperature sensor, a pH-indicator glass electrode and a reference electrode. The following cell is required for the pH measurements: glass electrode│test solution║salt bridge solution│reference electrode, (2) where the single vertical bar is the electrode-solution interface, and the double vertical bar is the liquid junction denoting an interface between the test solution and the salt bridge solution. Glass electrode may be designed in combination with an external reference electrode or as a single pH electrode. Glass electrode contains an internal 2 reference electrode (usually Ag/AgCl), an internal reference buffer (usually with pH 7) and a glass membrane. pH meter is basically a high impedance voltmeter with facility for converting the output voltage into pH, at measuring temperature, or at other chosen temperature, by temperature compensation. Direct pH readings are possible after adjustment of a pH meter. According to the International Vocabulary of Basic and General Terms in Metrology [3], adjustment is an operation of bringing a measuring instrument into a state of performance suitable for its use. For a pH meter adjustment, at least two so-called technical buffers are necessary [2]. If the electromotive force (emf) values corresponding to the two technical buffer solutions S1 and S2 with pHS1 and pHS2 are E1 and E2, respectively, the pH value of the unknown (test) solution can be calculated from its measured E by the following formula: pH pH S1 (E E1 ) pH S1 pH S2 E1 E 2 pH S1 E E1 . k' (3) where k' is the practical slope of the glass electrode [4]: k' E1 E 2 E pH pH S1 pH S2 (4) 3 The practical slope k' usually differs from the theoretical slope (Nernst coefficient) k = 2.3026 RT/F [5] (R is the universal gas constant, 8.31451 J·mol-1·K-1; T is the Kelvin temperature, F is the Faraday constant, 96485.309 Coulombs·mol-1). The pH value to be determined should lie between the pH values of two adjacent buffer solutions, i.e. the pH value of one buffer solution pHS1 shall be greater than that of the sample to be measured, while the pH value of the other buffer solution (pHS2) shall be less. Similar to experimental determination of other physical and chemical properties, pH measurement is affected by the limited accuracy of the measurement process. Some factors, such as liquid junction potential of cell (2), asymmetry potential of glass electrode, clogging of glass electrode diaphragm etc., lead to systematic errors in measured results, which can not be compensated by the adjustment procedure. Therefore, the accurate pH measurement requires a calibration of the pH meterelectrodes assembly [4]. Calibration is a set of operations that establish, under specified conditions, the relationship between values of quantities indicated by a measuring system, or values represented by reference materials, and the corresponding values realized by standards [3]. To achieve international comparability IUPAC recommended in 2002 to apply the traceability concept to pH measurements that will allow to connect pH measurement results from the national metrological institution level down to field, laboratory and industry measurements [6]. Traceability is a property of the result of a measurement or the value of a standard, whereby it can be related to stated references - usually national or international standards - through an unbroken chain of comparisons, all having stated 4 uncertainties. The unbroken chain of the comparisons (calibrations) is called a traceability chain [3]. In Israel, the National Physical Laboratory (INPL) is the national metrological institute. The national primary standard of pH value developed at INPL consists of a precise pH/ion-meter and the following electrochemical cell: Ag/AgCl│3 M KCl║3 M KCl║Test solution│H2 Pt/Pt, (5) where the pH-indicator electrode is the Pt/Pt hydrogen gas electrode; the reference electrode is Ag/AgCl│3 M KCl; the bridge solution is 3 M KCl. The primary standard is the standard that is designated or widely acknowledged as having the highest metrological qualities, and whose value is accepted without reference to other standards of the same quantity [3]. The emf of cell (5) depends on the activity of hydrogen ions according to the Nernst equation: E = EAg/AgCl - k·lg aH, (6) where EAg/AgCl is potential of the Ag/AgCl electrode, V. Calibration of cell (5) is carried out using six primary pH buffers at 25°C [7]. These buffers are prepared from the NIST standard reference materials (RMs) with expanded uncertainties of ± 0.005 pH, which are minimally known today. The national standard of pH value has successfully participated in two interlaboratory international key comparisons of pH measurement results in 2002 and 5 2004 organized by the Consultative Committee for Amount of Substance (CCQM) at the Bureau International des Poids et Mesures (BIPM). The described national standard of pH value is used for calibration of pH meterelectrode assemblies. The calibration consists of the following steps: 1) estimation of electrical resistance RE of the tested glass electrode; 2) calibration of the tested glass electrode; 3) calibration of the pH meter; 4) calibration of the temperature compensator. Electrical resistance of the glass electrode is determined as the slope of the voltammogram (current vs. potential) within the range of 0.0 ÷ -1.0.V. The RE should be not higher than 1109 Ohm. Calibration of the glass electrode is performed by measuring emf values with this electrode simultaneously with the reference hydrogen electrode in the same cell and buffer solutions. The experimental slope SlH of the linear calibration curve “E-pH” for buffers solutions measured with hydrogen electrode, and the similar slope SlG of the tested glass electrode, are determined by the regression analysis of all results of the measurements for each electrode. The SlH and SlG values should be not less than 53 mV/pH and not greater than 59.5 mV/pH at 25o C [8]. The ratio (SG / SH)100% should be not less than 90%. In order to make a pH meter calibration independent of the vagaries of electrode performance, the calibration is conducted by the pH meter comparison with known d.c. potentiometer [4] traceable to the national standard of volt. Potentiostat/galvanostat is using as d.c. voltage source in the range of applied potentials difference ± 2000 mV. 6 Calibration of a temperature compensator is done by comparing temperature values measured using this compensator with the readings of the reference thermometer traceable to the national temperature standard. The national primary standard of pH is used also for certification of buffers for pH measurements [9]. Thus, we are offering the following services based on the national primary standard of pH value and other INPL standards: Calibration of a pH-meter (as a voltmeter). Calibration of an automatic temperature compensator (as a thermometer). Calibration of a glass electrode. Certification of buffers for pH- measurements. These calibrations ensure the shortest unbroken traceability chain to the national standard of pH value and other INPL standards as required by ISO 17025 [10] for calibrations of any measurement instrument. References 1. P. Spitzer, B. Werner. Improved reliability of pH measurements. Analyt. and Bioanalyt. Chem. 2002, v. 374, pp. 787-795. 2. Measurement of pH value of clear aqueous solutions. German Standard DIN 19268, 1985. 3. International Vocabulary of Basic and General Terms in Metrology. 2nd ed., International Organization for Standardization, 1993, Switzerland. 7 4. Specification for Laboratory pH meters. British Standard 3145:1978, 1987. 5. F.G.K. Baucke. Thermodynamic Origin of the Sub-Nernstian Response of Glass Electrodes. Anal. Chem. 1994, v.66, pp.4519-4524. 6. R.P. Buck, S. Rondinini, A.K. Covington, F.G.K. Baucke and the others. The Measurement of pH – Definition, Standards and Procedures. Report of the Working Party on pH. IUPAC Provisional Recommendation. See http://www.iupac.org/reports/provisional/archives.html. or Pure & Appl. Chem. 2003, in press. 7. I. Ekeltchik, E. Kardash-Strochkova, O. Dreazen, and I. Kuselman. Influence of Buffers Quality on the pH Measurement Uncertainty: Prediction and Experimental Evaluation. Accreditation and Quality Assurance. 2002, v.7, pp. 412-416. 8. pH-Measurement; Supplementary pH-measuring Apparatus; Technical Requirements. German Standard DIN 19265, 1994. 9. I. Ekeltchik, E. Kardash-Strochkova, and I. Kuselman. In-house pH Reference Materials. Microchim. Acta. 2003, in press. 10. ISO/IEC 17025. General Requirement for the Competence of Testing and Calibration Laboratories. 1999.