MEASUREMENTS, CONVERSIONS AND DENSITY

MEASUREMENTS, CONVERSIONS AND DENSITY
Purpose: In this experiment you will become familiar with some basic laboratory equipment and measurements. You will also become familiar with the Metric System and calculate density.
Materials: Meter stick, Calculator, Balance, Wooden Blocks.
Introduction:
A laboratory balance is used to obtain the mass of a wooden block. The balance is simple to use, delicate and expensive. There are several different kinds of balances used, so the selection depends on the precision required.
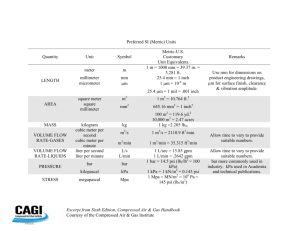
The meter (m) is the standard unit of measurement for length. The meter stick is divided into centimeters (1 cm = 0.01 m; 1m = 100 cm) and millimeters (1 mm = 0.001m;
1 m = 1000mm).
Density is a physical property that can be observed without altering the composition of a substance. Some physical properties enables us to identify and distinguish it from other substances.
Useful conversion factors:
1 lb = 453.6g
1 inch = 2.54 cm
PROCEDURE
It may be helpful to review SIGNIFICANT FIGURES
3.
B.
1.
Work in pairs or small groups
A.
1.
2.
Measurements
Obtain one of the wooden blocks and record it's number and density as listed on the block.
Study your ruler and evaluate the least distance between any two adjacent lines. This is called the least count . A good scientist (or student) will guess/estimate the distance between these lines to
1/4 to 1/5 of the least count. What is the least count on the ruler? ____________
3.
Take turns in measuring the sides of the wooden block and obtain an average for length, width and height. Each student should obtain a reading for the block and obtain an average measurement.
Mass Measurements
To make mass measurements use the mass balance. Study the divisions on the balance. What is the least count on the balance? __________
2. Obtain a balance and see if when empty it balances to zero. If it will not balance at zero obtain an
"empty reading" to be subtracted from the final reading. Each student should obtain a mass value for the block and obtain an average mass. If you need help, ask your instructor.
Each student should obtain a mass value for the block and obtain an average mass
Name_____________________
_____________________
_____________________
_____________________
_____________________
Date _____________________
MEASUREMENTS, CONVERSIONS AND DENSITY
REPORT SHEET
Block #__________
Least count on the metric ruler __________
Least count on the balance _____________
Length___ Height
Listed density____________lb/ft 3
Width Mass
6)
5)
7)
4)
3)
2)
1)
________cm
________
________
________
________
Average ________cm
Calculate the VOLUME = L x W x H a)
________cm
________
________
________
________
________cm
Calculate the density = __________ (units?)
________cm
________
________
________
________
________
________cm in centimeters (using a calculator) __________ (units?)
Convert the mass in grams to English pounds
________ g equals
Convert the volume from cubic centimeters to cubic feet
________ cc
Calculate the density in 1b/ft equals
Compare the calculated density to the value listed on the block
Calculate your percent error
%
3 ________
Error = I calculated density - listed density I x 100 lb ft 3
listed density
________g
________
________
________
________
________g