measurement uncertainty
advertisement

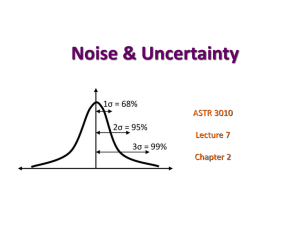
ARG/PDW: MCEN4027F00 I: 1 MEASUREMENT UNCERTAINTY Introduction The goal of experimentation is to determine the behavior of physical systems. In conducting experiments we utilize measurement systems that interact with the physical system being studied such that we are able to estimate the value of physical parameters of interest. We then use the measurement results to test hypotheses about the physical systems. In order to test hypotheses and draw conclusions we must be able to assess the quality of the measurements, i.e., we must be able to estimate the extent of any error in the measurements. All experimental measurements contain some error. Fixed (systematic) errors are associated with repeated readings that are in error by the same amount. Random errors usually follow some statistical distribution. Gross (illegitimate) errors are associated with invalid data. In general, it can be difficult to distinguish between fixed and random errors. ARG/PDW: MCEN4027F00 I: 2 Classification of Measurement Errors Systematic or Fixed Errors Calibration errors Consistently occurring human error Errors of the measurement instrument Consistent calculation errors Uncorrected errors due to the presence of the measurement instrument Random Errors Errors due to uncontrolled environmental variations Inconsistently occurring human errors Errors derived from a lack of sensitivity Illegitimate (Gross) Errors Mistakes Computational errors Extreme events (failure) ARG/PDW: MCEN4027F00 I: 3 Important Concepts The quality of measurements is characterized by the concepts of precision, accuracy and bias. Precision is the closeness of repeated measurements of the same quantity. A measurement with small random errors is said to have high precision. Accuracy is the closeness of a measured value to its true value. A measurement with small error of any kind is said to have high accuracy. A measurement with small systematic errors is said to be unbiased. Calibration is the process followed to remove bias and define precision. Calibration is performed by employing a system that produces a known value of a given parameter (standard). ARG/PDW: MCEN4027F00 I: 4 Consequences A biased but sensitive scale might yield inaccurate but very precise weight. An insensitive scale might result in an accurate although imprecise reading since a repeated weighing would be unlikely to yield an equally accurate weight. Unless there is bias in a measuring instrument increasing precision will lead to an increase in accuracy. ARG/PDW: MCEN4027F00 I: 5 Significant Figures The last digit of an approximate number should always be significant, i.e., it should imply a range for the true measurement corresponding to a "unit step" above and below the recorded value. For example, 193 implies 192.5 - 193.5 192.8 implies 192.75 - 192.85 192.76 implies 192.755 - 192.765 Note that these three numbers are given in order of increasing accuracy. This scheme applies to all digits including zero. For example, 7.80 implies the limits 7.795 - 7.805; if 7.75 7.85 is implied, then the measurement should be recorded as 7.8. Zeros should not be written at the end of approximate numbers to the right of the decimal point unless they are meant to be significant digits. Rounding This is a process to reduce the number of significant figures. The general rules result in: Original Number Significant Figures Desired Result 26.68 2 27 133.7137 5 133.71 0.03725 3 0.0373 0.03715 3 0.0372 18316 2 18,000 ARG/PDW: MCEN4027F00 I: 6 Expressing Uncertainty in Measurements Suppose we are to perform a measurement of a quantity, y. In all experiments there is some imprecision, and thus the measurement could be in error. The uncertainty is our statement as to the probability of any single measurement being in error. The statement is generally made in the form, yw @ 1 100% where y is the instrument reading, w is half of the confidence interval (symmetric), and 1- is the confidence level usually expressed in percent. If we knew the distribution function that described variability in the instrument, we could be precise about w for a given confidence level. Ideally the confidence interval is obtained from calibration. ARG/PDW: MCEN4027F00 I: 7 In a calibration experiment, we attach the instrument to a known, stationary source (standard). A sequence of readings from the instrument is then taken, and the statistical parameters of the distribution are calculated. The sample mean is used to correct the measurement for bias and the sample variance is used to estimate the confidence interval of a single measurement. The bias-corrected result is then presented as, y t 2, S n @ 1 100% where S is the calibration standard deviation and n = 1 is used for a single reading. Note that t/2, depends upon the confidence level and the number of readings used for S. Typically an of 0.05 is used leading to a confidence level of 95%. I: 8 Measured Value ARG/PDW: MCEN4027F00 Perfect Calibration True Value Measured Value Systematic Error One-Point Calibration Correction True Value ARG/PDW: MCEN4027F00 I: 9 Measured Value Systematic Error (Linear) Two-Point Calibration Correction True Value Measured Value Systematic Error (Non-Linear) Multiple-Point Calibration Correction True Value ARG/PDW: MCEN4027F00 I: 10 If uncertainty is limited by resolution rather than by variability, then a reasonable way to express the result of a measurement is, y Rm @ 1 100% where Rm is the resolution with which a 1- confidence level can be maintained. A good example of a resolution-limited instrument is a ruler with coarse markings. Guessing is the last resort for determining instrument uncertainty. In practice, one is usually forced to make a conservative estimate for w rather than risk drawing inappropriate conclusions from the data. Results should be reported as, yw @ 1 100% and a statement made that the uncertainty has been estimated. ARG/PDW: MCEN4027F00 I: 11 Propagation of Uncertainty The above discussion applies to the description of uncertainty in a measured quantity. In practice, directly measured quantities are usually used to calculate a secondary quantity. If the uncertainty in a directly measured quantity is known, how is the resulting uncertainty in the calculated quantity determined? Suppose we calculate a result R that is a function of individual measurements of different quantities x1, x2, ……. xn, Then, R Rx1, x2 ,....xn and statistical analysis can be used to show that if the x’s are each independent and normally distributed, 2 n R wR w j j 1 x j Here, wR is the uncertainty in the calculated quantity. In some circumstances the fractional uncertainty, wR/R, is more useful and can be represented by, wR R ln R w j j 1 ln x j x j n 2 ARG/PDW: MCEN4027F00 I: 12 Once the uncertainty for the calculated result is known, the value is then expressed as, R wR @ 1 100% and for fractional uncertainty, R wR R @ 1 100% It is important to keep in mind that the uncertainty analysis results in a statement regarding the variability introduced by the measurement system due to “noise” or resolution limits not variability in the sample or sample population under study. ARG/PDW: MCEN4027F00 I: 13 EXAMPLE A resistor has a nominal stated value of 10 1 percent. A voltage is impressed on the resistor, and the power dissipation is to be calculated in two different ways: (1) from P = E2/R and (2) from P = EI. In (1) only a voltage measurement will be made, while both current and voltage will be measured in (2). Calculate the uncertainty in the power determination in each case when the measured values of E and I are: E = 100 V 1 % (for both cases) I = 10 A 1 % ARG/PDW: MCEN4027F00 I: 14 SOLUTION Using the schematic shown, for the first case we have: P 2 E ; E R P E2 2 R R and applying the basic equation, 2 2 2 2 E E 2 2 wP wE 2 wR R R 12 Dividing by P = E2/R gives 2 2 wP wE wE 4 P E R 12 Inserting the numerical values for uncertainty, 12 wP 40.012 0.012 2.236% P For the second case we have, P I; E P E I and after appropriate algebraic manipulation, we obtain 2 2 wP wE wI P E I 12 ARG/PDW: MCEN4027F00 I: 15 Once again, inserting the numerical values for uncertainty, wP 2 212 0.01 0.01 1.414% P Thus, the second method of power determination provides considerably less uncertainty than the first method, even though the primary uncertainties in each quantity are the same. In this example the utility of the uncertainty analysis is that it affords the individual a basis for selection of a measurement method to produce a result with less uncertainty.