MINERALOGY OF THE SILICATES
advertisement
34
MINERALOGY OF THE SILICATES
Silicon, after oxygen, is the most abundant element in the Earth’s crust and mantle,
and because the Si-O bond is considerably stronger than any other metal – oxygen
bond, silicate minerals make up the vast majority of rocks. 95% of the earth’s crust is
made up of only a handful of mineral groups, and the rest of these lectures will be
concerned with their structure and composition.
The basis for silicate structures is the Si – O bond, and the [SiO4] tetrahedron. In
almost all silicate minerals Si is tetrahedrally coordinated by oxygen, with the O-Si-O
bond angle only slightly deviating from the ideal value of 109.5o. Although the Si-O
bond is approximately 50% ionic and 50% covalent, it is convenient to consider the
silicon as tetravalent Si4+ and the oxygen as O2-, so that the net charge of an isolated
tetrahedron is [SiO4]4-. The [SiO4] tetrahedron is the building unit of all silicate
structures. Tetrahedra can be combined with other in several ways, showing
increasing degrees of polymerisation, from isolated tetrahedra, pairs of tetrahedra,
single chains, double chains, sheets and continuous frameworks. Tetrahedra are
always connected to each other by corner-sharing, but no more than two tetrahedra
can share a common corner, i.e. a bridging oxygen.
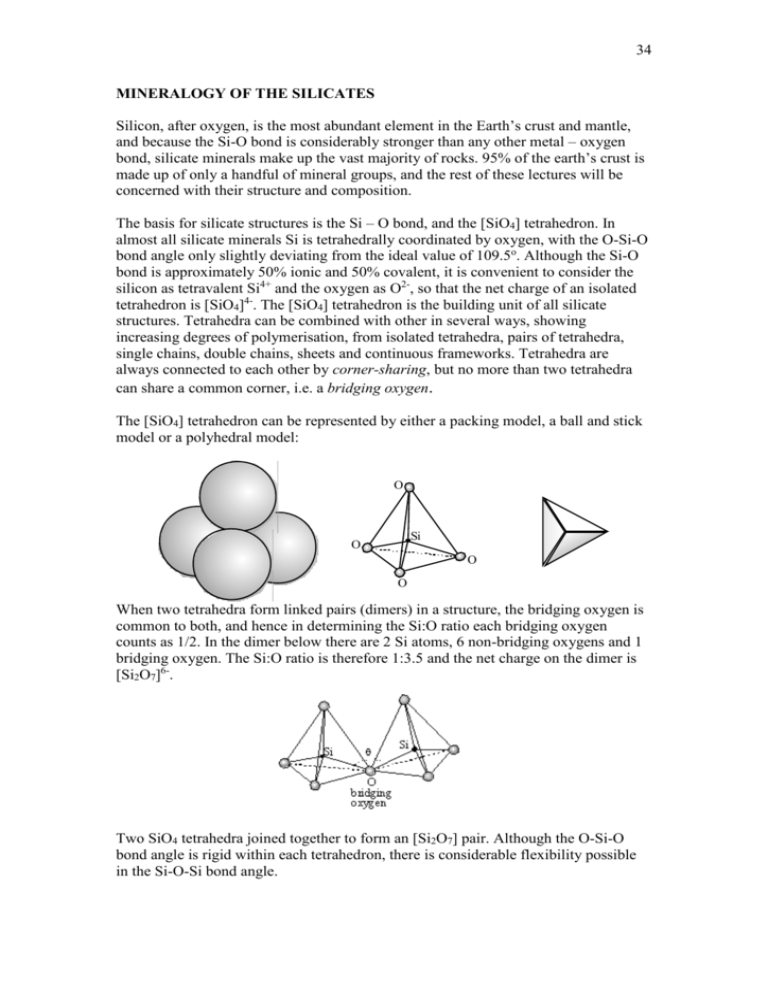
The [SiO4] tetrahedron can be represented by either a packing model, a ball and stick
model or a polyhedral model:
O
Si
O
O
O
When two tetrahedra form linked pairs (dimers) in a structure, the bridging oxygen is
common to both, and hence in determining the Si:O ratio each bridging oxygen
counts as 1/2. In the dimer below there are 2 Si atoms, 6 non-bridging oxygens and 1
bridging oxygen. The Si:O ratio is therefore 1:3.5 and the net charge on the dimer is
[Si2O7]6-.
Two SiO4 tetrahedra joined together to form an [Si2O7] pair. Although the O-Si-O
bond angle is rigid within each tetrahedron, there is considerable flexibility possible
in the Si-O-Si bond angle.
35
Increasing silicate polymerisation.
Single chain of tetrahedra, all pointing in one direction.
Each tetrahedron shares two corners. The formula of the
repeating unit (outlined) is [Si 2O6]4-
Double chain of tetrahedra, with their bases all in a common
plane and all pointing up. Halfthe tetrahedra share two corners,
the other half share three corners. The formula of the repeating
unit (outlined) is [Si 4O11]6-
Part of a continuous sheet of tetrahedra, with their bases all in
a common plane and all pointing up. Each tetrahedron shares
three corners. The formula of the repeating unit (outlined) is
[Si 2O5]2-
In silicate framework structures each silicate tetrahedron is connected by all four corners
to other silicate tetrahedra, so that all oxygen atoms are corner-sharing. The Si:O ratio is
therefore 1:2 and there is no net charge on the whole framework. The mineral quartz,
SiO2 is a framework silicate.
In most framework structures some Al3+ substitutes for Si4+ in the tetrahedra, which
gives the framework a net negative charge and so requires other cations in the structure to
balance the charge. The Al – Si substitution is a very important phenomenon in minerals.
MINERALS WITH ISOLATED SiO4 TETRAHEDRA (ORTHOSILICATES)
In this group isolated [SiO4] tetrahedra are linked together by other cations wich lie
between them. To produce an electrically neutral structure the charge on each tetrahedron
has to be balanced by 4 positive charges. These metal ions such as Fe2+, Mg2+, Ca2+ etc.
link the tetrahedra together.
36
The most important mineral in this group is
Olivine
The name ‘olivine’ refers to a continuous range of compositions between Mg2SiO4
(forsterite) and Fe2SiO4 (fayalite) and we usually write the formula as (Mg,Fe)2SiO4,
indicating that Mg and Fe can substitute for each other. This type of continuous chemical
mixing is called a solid solution and is a very common phenomenon in minerals. The
most important factor which controls solid solution formation is the relative size of the
cations.
The olivine structure.
The left diagram above shows the arrangement of [SiO4] tetrahedra in projection down
the a axis of the orthorhombic unit cell (dashed line). The isolated tetrahedra point
alternately up and down along rows parallel to the c axis. In this projection there are rows
at two levels in the unit cell, the lower level (at a=0) drawn in a heavier line and an upper
level (at a = 1/2). Within each level the tetrahedra are linked by octahedra which contain
the the cations (termed M sites, and shown by the small circles).
In the right hand diagram the lower level is shown in the same projection. This shows
how the octahedra are linked together. There are two kinds of octahedra, labelled M1 and
M2. The M1 octahedra share edges to form ribbons parallel to the c axis. Ribbons in one
layer are connected to those in the layer above by the M2 octahedra, also by edgesharing.
Other olivine compositions.
37
In the Fe-Mg olivines the Fe and Mg ions are randomly mixed over the M1 and M2 sites.
In the Ca-bearing olivines the Ca is in the M2 sites while Fe and Mg are mixed over the
M1 sites.
There is virtually no solid solution between the Ca-bearing olivines and Fe,Mg olivines
because the Ca ion is too big to substitute into M2 sites when they are occupied by
Fe,Mg.
At higher temperatures the structure expands by increasing the size of the M sites, and so
more solid solution is possible. At 10000C there is about 5% substitution of Ca for
Fe,Mg; at 1450oC it is 20%. This is a general and important principle in Mineralogy.
Occurrence of olivine in rocks.
Olivine crystallises at very high temperatures from melts that are rich in metals and
relatively poor in silica. Olivine is one of the first minerals to crystallise as a melt
(magma) cools and is often found in well-formed crystals, surrounded by latercrystallising minerals.
The temperature at which olivine crystallises depends on the composition. This can be
seen in the phase diagram in the next section.
The olivine phase diagram.
The melting point of forsterite is 1890oC and fayalite is 1205oC. Only the pure endmembers of the solid solution melt at fixed temperatures. Solid solutions melt over a
range of temperatures as shown in the phase diagram below. Similarly, only pure melts
begin to crystallise solids of the same composition as the melt. All other melt
compositions crystallise solids more Mg rich than the melt.
38
When a melt of composition Fo50 (i.e. 50 mole% Forsterite) cools the first crystals to
form, at ~1720oC have a composition of Fo80 (shown by the horizonal tie line from the
composition on the liquidus to the composition on the solidus). If equilibrium is
maintained, the first-formed crystals react with the melt as cooling continues, so that at
any temperature the equilibrium compositions of the melt and the crystals are given by
horizontal tie lines. Crystallisation is complete at 1500oC when the final olivine crystals
will have the same composition as the initial melt. At temperatures between 1720oC and
1500oC, both the compositions of coexisting olivine + melt and their proportions change.
If equilibrium is not maintained during the cooling process the olivine crystals will be
compositionally zoned with cores that are more Mg rich than the margins. This happens if
cooling is too fast for the crystals to reequilibrate with the melt.
Melting is the reverse of crystallisation. A crystal of composition Fo50 begins to melt at
1500oC and the first liquid to form has composition Fo20.
Phase transitions in Mg2SiO4 in the earth.
Mg2SiO4 exists as the olivine structure in the earth down to a depth of 400km when the
high pressure tranforms the olivine structure to the spinel structure with the same
composition. This is termed a phase transition, and the study of such structural changes is
one of the most important aspects of Mineralogy. The spinel structure of Mg2SiO4 is
about 6% denser than the olivine structure and so is more stable at high pressure. The
formation of this denser structure is responsible for sharp discontinuity (increase) in the
velocity of seismic waves at 400km depth. The transition is also thought to trigger deep
earthquakes because of the volume reduction.
Garnet group
The garnets are more complex both chemically and structurally than olivines. The general
formula of garnets is A32+B23+(SiO4)3 where A is Ca2+, Mg2+, Fe2+ or Mn2+ and B is Al3+,
Fe3+ or Cr3+. Thus there is a much wider range of possible compositions of garnets than
olivines. The structure is cubic and consists of isolated silicon tetrahedra bonded by BO6
octahedra by corner sharing, while the larger A cations have 8-fold distorted cubic
coordination.
39
Part of the garnet structure showing the linkage between the isolated SiO4 tetrahedra and
the BO6 octahedra. The large A sites are left unshaded. Only the O atoms (small circles)
are shown.
Garnet compositions
Natural garnets are commonly divided into two groups – those in which the A cation is
Ca2+ (the grandite group) and those in which the A cation is not Ca2+, but the B cation is
Al3+ (pyralspite group).
Grandite group
Pyralspite group
Grossular
Andradite
Uvarovite
Pyrope
Almandine
Spessartine
Ca3Al2(SiO4)3
Ca3Fe3+2(SiO4)3
Ca3Cr2(SiO4)3
Mg3Al2(SiO4)3
Fe2+3Al2(SiO4)3
Mn3Al2(SiO4)3
Within the grandite group there is a continuous solid solution between grossular and
andradite. Within the pyralspite group there is extensive substitution of Mg, Fe and Mn in
the A site, although the compositions of natural garnets tens to fall either between pyrope
and almandine, (Mg,Fe2+3)Al2(SiO4)3 or between almandine and spessartine,
(Fe2+3, Mn)Al2(SiO4)3. It is also possible to find garnets in high temperature metamorphic
rocks (formed above 700oC) which have compositions inetermediate between the two
groups, i.e. (Ca,Mg,Fe2+3)Al2(SiO4)3.
Zircon
Zircon, ZrSiO4 , is an important industrial mineral because it is the main source of
zirconium Zr, used in nuclear reactors and zirconia ZrO2, an important ceramic material.
It is found in small amounts in many different types of rocks, and usually only as
microscopic crystals. However as it is very dense and resistant to weathering, it can form
significant concentrations in sand deposits from which it is easily separated.
Zircon also almost always contains some hafnium as well as uranium and thorium. The
radioactive decay of uranium to lead provides the basis for radiometric dating of rocks.
The radioactivity also damages the crystal structure and makes it metamict (another word
for amorphous, but used in this special sense).
Zircon is tetragonal and most grains form small tetragonal prisms. The zircon structure
consists of isolated [SiO4] tetrahedra (dark in the following diagram) with the large Zr
ions (as well as the other minor elements) in 8-fold coordination.
40
The zircon structure
The Aluminium Silicates Al2SiO5.
The three polymorphs of Al2SiO5, andalusite, kyanite ans sillimanite are very important
minerals in metamorphic rocks. They are often referred to as the aluminosilicate
polymorphs. When the formula is written as AlAlO(SiO4) it is clear that the minerals
belong to the orthosilicates and have two different Al structural sites as well as isolated
[SiO4] tetrahedra. This is also clear from the crystal structures.
The structures of the three polymorphs share a number of common features. In all three
minerals straight chains of edge-sharing AlO6 octahedra extend along the c axis. These
octahedra contain half of the Al in the structural formula. The remaining Al atoms are in
coordination which is different in each mineral: 6-fold sites in kyanite, 5-fold sites in
andalusite and 4-fold sites in sillimanite. These Al-polyhedra alternate with [SiO4]
tetrahedra, also along the c axis, linking together the AlO6 chains.
Our main interest in these minerals will be in their relative stability fields as a function of
pressure and temperature. Kyanite is 14% denser than andalusite and 11.5% denser than
sillimanite, so that on this basis we would expect kyanite to be stable at the highest
pressures and lowest temperatures. Andalusite is the low pressure phase and sillimanite is
stable at high temperatures and moderate pressures.
41
Andalusite
Sillimanite
Kyanite
Approximate P-T stability fields of the three polymorphs.
42
SINGLE CHAIN SILICATES
By far the most important minerals in which the [SiO4] tetrahedra are linked to form a
linear single chain are the pyroxenes.
The basic pyroxene structure is shown below.
(a) A single pyroxene chain which extends along the c axis and below, a schematic
representation of this chain viewed end-on. (b) The arrangement of SiO4 chains in the
pyroxene structures, viewed along the c axis. The M1 cations form chains of edge-sharing
octahedra between the apices of the tetrahedra, while the larger M2 octahedra form
similar chains between the bases of the tetrahedra.
All the pyroxenes have this basic structure, but deepending on the compositions there are
variations in the size of the M octahedra and this affects the way in which the tetrahedra
link to the octahedra. When the M sites have their largest possible size, the tetrahedral
chain is straight, as shown above. As the M sites become occupied by smaller cations the
tetrahedral chain must become shorter to accommodate this. This can only happen by
kinking the tetrahedral chain, or by changing the arrangement of chains along their length
i.e. moving the chains relative to one another along the c axis.
43
In the figure above the tetrahedral chain has to be rotated to fit with the octahedral M
chain, which has been shortened by 4%. This happens when the M sites are occupied by
smaller cations or as the temperature decreases.
Pyroxene compositions.
(a)
Calcic and Fe,Mg pyroxenes.
The shaded areas represent the extent of solid solution in naturally occurring pyroxenes. At high
temperatures there is complete solid solution between augite and pigeonite.
The Ca-rich pyroxenes, as well as pigeonite are monoclinic, and are termed
clinopyroxene. The Ca is always in the larger M2 site. In Ca-rich clinopyroxenes the
chains are straight, whereas in pigeonite the chains are rotated and shortened to better
accommodate the smaller Fe,Mg cations. When there is very little or no Ca present, the
M2 site becomes even smaller, and this is done by arranging the chains differently along
their length. The Ca-poor structures are orthorhombic and are termed orthopyroxene.
44
(b)
Sodic and sodic-calcic pyroxenes.
NaAlSi2O6
NaFe 3+ Si2O6
jadeite
omphacite
aegerin e
aegerin eaugite
CaAl(Al,Si) 2O6
calcic
clin op yro xene
Ca(Mg,Fe)Si2O6
In high pressure rocks pyroxenes are enriched in Al in octahedral coordination, as in
jadeite, which has Na in the M2 sites and Al in the M1 sites. Above about 700oC there is
complete solid solution between jadeite and augite, involving a coupled substitution:
Na+ + Al3+ Ca2+ + (Mg2+,Fe2+), thus maintaining the overall charge balance.There is
also some replacement of Al3+ by Fe3+. Omphacite forms below 700oC and is a distinct
mineral species because of the way in which the cations are arranged or ordered in the
M1 and M2 sites. For this reason omphacite has a lower symmetry than jadeite or augite,
but all these pyroxenes are still clinopyroxenes.
The augite-pigeonite phase diagram.
On the previous page it was stated that “at high temperatures there is complete solid
solution between augite and pigeonite” yet the composition field of naturally occurring
Ca,Mg,Fe pyroxenes does not show any compositions between augite and pigeonite. This
can be understood from the phase diagram, which shows the compositions of co-existing
phases as a function of temperature. It is more complex than the olivine phase diagram
because the solid solution is only complete at high temperatures and because there is a
phase transition from clinopyroxene to orthopyroxene in the Ca-poor pigeonite.
As we can see from the phase diagram pigeonite is only formed at very high temperatures
and should transform to orthopyroxene as the rock cools. However, this transformation
involves a complete reorganisation of the silicate chains (it is called a reconstructive
phase transition) and takes a very long time. So if a rock is very slowly cooled the
pigeonite is transformed to orthopyroxene, but in rapidly cooled rocks (such as volcanic
rocks) the pigeonite structure is preserved. Instead, the silicate chain in pigeonite distorts
and gets shorter. This is also a phase transition because it involves a change in the
structure, but in this case it is very easy and fast because it involves no bond-breaking.
Such a transition is called a displacive phase transition and is shown as the highpigeonite low-pigeonite transition in the schematic phase diagram below.
45
The high-low pigeonite transition is shown as a dashed line because it is not strictly part
of the equilibrium phase diagram. Low pigeonite is metastable (not thermodynamically
stable butdoes not transform to opx for kinetic reasons.)
Exsolution
The decomposition of a solid solution to form a two-phase intergrowth occurs by a solid
state diffusion process and is called exsolution or phase separation. The intergrowth is
usually on a very fine-scale because diffusion is a slow process. Even in very slowly
cooled rocks an optical microscope is needed to see the two phases, and in more rapidly
cooled rocks the intergrowth is even finer and an electron microscope is needed to see it.
The transformation of pigeonite to orthopyroxene also results in exsolution because the
orthopyroxene can contain less Ca than pigeonite. Therefore orthopyroxene very often
contains exsolution lamellae of augite as a result of the transformation.
Because solid solutions are common in many mineral groups, and the compositional
range of solid solutions is greater at high temperatures, exsolution is a common process
during cooling in rocks. The scale of the exsolution intergrowth (often termed the
microstructure) is an indicator of the cooling rate of the rock.
STRUCTURE AND CLEAVAGE
The way a crystal breaks depends on the crystal structure. Glass, for example, breaks in
an irregular way, but crystals often break along planes of weakness, leading to perfectly
planar fractures called cleavage planes. Cleavage planes in individual minerals can be
seen on broken surfaces of rocks, and also in thin rock sections prepared for optical
microscopy. Because of the relationship between cleavage and structure, the cleavage
planes present in a mineral are characteristic and diagnostic.
46
The strong structural units in pyroxenes are the groups opposing tetrahedral chains
bonded by the M1 sites between them. These units are shown in the diagram below as “Ibeams”, and the cleavage planes in pyroxenes pass between these I-beams. On the atomic
scale these cleavage planes are not flat, but on average they form cleavages at
approximately 90o to each other. The cleavage planes are also shown in the drawing of a
single crystal of pyroxene.
(a) The I-beam representation of the pyroxene structure. Each pair of opposing chains
together with the M1 sites is shaded and represents an “I-beam”, extending along the c
axis. Two sets of cleavage planes at approx 90o are shown. (b) A crystal of pyroxene
showing the traces of the cleavage planes on the crystal faces. The cleavage planes are
parallel to the set of {110} planes in the crystal.
DOUBLE CHAIN SILICATES – the AMPHIBOLES.
The structure of the amphibole minerals is similar to the pyroxenes, but based on double
rather than single chains. There are also similarities in the way the structure behaves as a
function of chemical substitutions, temperature and pressure. Thus there are
clinoamphiboles when larger cations such as Ca and Na are present, and orthoamphiboles
when only smaller cations such as Fe and Mg are present. The double chain can also be
shortened by rotation of the tetrahedra to accommodate smaller cations – this happens in
cummingtonite just as it does in pigeonite. The solid solution between Ca-rich and Capoor amphiboles also becomes more restricted as the temperature decreases, resulting in
exsolution intergrowths.
47
Amphiboles have a wider range of compositions than pyroxenes and also contain
hydroxyl groups as essential consituents.
A double chain of SiO4 tetrahedra in amphiboles, extending along the c axis, and below,
a schematic representation of this double chain viewed end-on. Here the chains are
straight – in practice they are always slightly rotated. (b) The arrangement of double
chains in the amphibole structures, viewed along the c axis. The M1, M2 and M3 cations
form chains of edge-sharing octahedra between the apices of the tetrahedra, while the
larger M4 octahedra form similar chains between the bases of the tetrahedra. The A sites
and the OH sites lie in the rings formed along the double chain. One I-beam has been
shaded.
Amphibole compositions.
The amphibole quadrilateral showing the approximate compositions of simple amphibole minerals. The
shaded areas represent the extent of solid solution in naturally occurring minerals. At high temperature
there is complete solid solution between cummingtonite and actinolite.
48
The general composition of amphibole minerals may be described by the formula
A0-1B2C5T8O22(OH,F)2.
A = the large A site which may be vacant or contain varying amounts of Na/Ca.
B = the content of the M4 site which in the most common amphiboles may be
Ca,Na,Fe2+ or Mg;
C = Fe2+,Mg, Fe3+ or Al in the M1,M2 and M3 sites,
T = Si and Al in the tetrahedra. The limit of this substitution appears to be (Al2Si6).
This large number of cation sites makes the amphiboles the mineral group with the widest
chemistry, and so able to crystallise in rocks over a broad range of bulk composition. A
typical complex solid solution is that of "hornblende" a very common constituent of
many igneous and metamorphic rocks. The composition of hornblende can be described
by a substitution scheme starting with tremolite Ca2Mg5Si8O22(OH)2. Al3+ can
substitute for Si4+ in the tetrahedral double chains, as well as for Mg in the octahedra. The
charge is balanced by Na substitution in the large A sites, giving a composition such as
NaCa2(Mg4Al)(Al2Si6)O22(OH)2. There is also always some substitution of Fe2+ and
Fe3+ giving ‘hornblende’ a very wide range of possible compositions.
In addition, at high pressures there is also Al substitution into the octahedral sites, charge
balanced by Na+. The sodium amphibole group is a solid solution with two end members:
glaucophane : Na2Mg3Al2Si8O22(OH)2 and
riebebeckite: Na2Fe2+3Fe3+2Si8O22(OH)2
Cleavage planes in amphiboles
The cleavage planes in amphiboles are exactly analogous to those in pyroxenes and pass
between the I-beams. Because of the greater width of the double chain, the cleavage
planes are now at approx 600 to each other.
The cleavage planes are parallel to the set of {110} planes in the crystal.
~60o
001
010
110
110
49
SHEET SILICATES
The sheet silicates form a large number of different minerals, including mica and trhe
clay minerals, and they share the same basic building blocks : a tetrahedral sheet, and one
of two kinds of octahedral sheet. The sheets are combined to form composite structures
e.g. an octahedral sheet sandwiched between two tetrahedral sheets.
(a) Part of an infinite tetrahedral sheet,with SiO4 tetrahedra all pointing in the same
direction.
(b) Part of an infinite sheet of edge-sharing octahedra. In this case all the octahedra are
occupied and it is called a trioctahedral sheet. The octahedra are occupied by divalent
ions such as Mg.
(c) When the octahedra contain trivalent ions such as Al3+ one third remain unfilled, and
the remaining octahedra become distorted. Such a sheet is called dioctahedral.
The formula for the tetrahedral sheet is [Si2O5]. The ideal formula for a trioctahedral
sheet is Mg3(OH)6, the same as the mineral brucite, which has a structure of trioctahedral
sheets weakly held together with van der Waals bonds. The ideal dioctahedral sheet has a
formula Al2(OH)6 which is the same as the mineral gibbsite.
The way in which a tetrahedral sheet links to an octahedral sheet is shown below.
Note that for this to be possible the repeat distances in both sheets must be compatible. If
50
the octahedra are too small, the tetrahedra need to rotate within the sheet to achieve a
better fit. Thus the diagram above is rather idealised.
There are three main groups of layer silicate minerals.
In the 1 : 1 layer silicates, the layers are made up from one sheet of [SiO4] tetrahedra,
combined with one octahedral sheet. These layers are charge balanced and only weak
bonds hold successive layers together in the stack.
In the 2 : 1 layer silicates, an octahedral sheet is sandwiched between the apices of two
tetrahedral sheets. These Tet–Oct–Tet layers are either held together by weak van der
Waals forces if they are neutral (e.g. as in talc) , or may have cations between them for
charge balance, if substitutions in either sheet result in a residual layer charge (e.g. as in
the micas).
In the 2 : 1 : 1 layer silicates an additional octahedral sheet is sandwiched between each
T–O–T layer.
51
Compositions of 1:1 Layer silicates
e.g. kaolinite:
dioctahedral
e.g serpentine
trioctahedral
1 T sheet + 1 O sheet = TO layer + Hydroxyl
Si2O52- + Al2(OH)6 = Al2Si2O5(OH)4 + 2(OH)1 T sheet + 1 O sheet = TO layer + Hydroxyl
Si2O52- + Mg3(OH)6 = Mg3Si2O5(OH)4 + 2(OH)-
Compositions of 2:1 Layer silicates
(i) with no interlayer cations
e.g pyrophyllite
dioctahedral
2 T sheets + 1 O sheet = TOT layer + Hydroxyl
2(Si2O52-) + Al2(OH)6 = Al2Si4O10(OH)2 + 4(OH)-
e.g talc
trioctahedral
4(OH)-
2 T sheets + 1 O sheet = TOT layer + Hydroxyl
2(Si2O52-) + Mg3(OH)6 = Mg3Si4O10(OH)2 +
The TOT layers are electrically neutral and so don’t need any additional interlayer cations
to hold them together. They are very weakly bonded by van der Waals bonding, and this
is why both are very soft and feel waxy.
(ii) with interlayer cations
One quarter of the Si4+ in the tetrahedral sites is replaced by Al3+ giving the layer a net
negative charge. This is compensated by interlayer cations.
e.g. muscovite dioctahedral
TOT layer + interlayer cation
Al2(AlSi3O10)(OH)2- + K+ = K Al2(AlSi3O10)(OH)2
e.g. phlogopite trioctahedral
TOT layer + interlayer cation
Mg3(AlSi3O10)(OH)2- + K+ = K Mg3(AlSi3O10)(OH)2
When some Fe2+ substitutes for Mg the result is the common mica mineral
biotite - K (Mg,Fe)3(AlSi3O10)(OH)2
Compositions of 2:1:1 Layer silicates
The most common mineral in this group is chlorite which can be thought of in its
simplest form as a TOT talc layer with a brucite sheet in between, i.e.
Chlorite - dioctahedral - Mg3Si4O10(OH)2.Mg3(OH)6