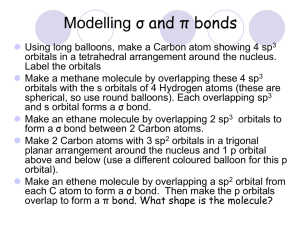
HW-9
advertisement

Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals INSTRUCTOR’S NOTES This chapter is a natural complement to the previous chapter’s introduction to bonding and related subjects. The emphasis in this chapter is on valence bond theory, with only about one and a half lectures on molecular orbital theory. Although MO theory is not always included in general chemistry, its wide application in organic chemistry argues for at least minimally addressing the topic. The consistent answers for molecular geometry which arise from VSEPR and valence bond theory is a good point to make. There are many molecular modeling programs that can be used to build molecular models and hybrid and molecular orbitals such as the CACheTM software mentioned in the previous chapter’s notes. About three or four lectures are scheduled for this chapter. SUGGESTED DEMONSTRATIONS 1. Orbital Overlap For an overhead demonstration of orbital overlap, see Rothchild, R. “Efficiency of Orbital Overlap: Visual Demonstration,” Journal of Chemical Education 1981, 58, 757. 2. Hybrid Orbitals In these lectures it is most useful to show models of molecules with the hybrid orbitals in place. Suitable models can be obtained from Aldrich Chemical Company. Emerson, D. W. “A Colorful Demonstration to Simulate Orbital Hybridization,” Journal of Chemical Education 1988, 65, 454. Samoshin, V. V. “Orbital Models Made of Plastic Soda Bottles,” Journal of Chemical Education 1998, 75, 985. 3. Molecular Orbital Theory Shakhashiri, B. Z.; Dirreen, G. E.; Williams, L. G.; Smith, S. R. “Paramagnetism and Color of Liquid Oxygen: A Lecture Demonstration,” Journal of Chemical Education 1980, 57, 373. Saban, G. H.; Moran, T. F. “A Simple Demonstration of O 2 Paramagnetism. A Macroscopically Observable Difference Between VB and MO Approaches to Bonding Theory,” Journal of Chemical Education 1973, 50, 217. 174 See the ChemistryNow website for a demonstration of the paramagnetism of liquid oxygen. Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals SOLUTIONS TO STUDY QUESTIONS Cl 9.1 Cl C Cl H The electron-pair and molecular geometries are tetrahedral. The C atom is sp3 hybridized. Three of these hybrid orbitals each overlap with a chlorine 3p orbital to form three C—Cl sigma bonds. One hybrid orbital overlaps with a hydrogen 1s orbital to from a C—H sigma bond. F 9.2 F N F The electron-pair geometry is tetrahedral and the molecular geometry is trigonal pyramidal. The N atom is sp3 hybridized. Three of these hybrid orbitals each overlap a fluorine 2p orbital to form three N—F sigma bonds. 9.3 The nitrogen atom is sp3 hybridized. The bond is formed by overlap of a nitrogen sp3 orbital and an oxygen sp3 orbital. 9.4 The nitrogen atoms are sp3 hybridized. The bond between the nitrogens is formed by overlap of sp3 hybrid orbitals. 9.5 The electron-pair geometry and molecular geometry are both trigonal planar. The hydridization of the carbon atom is sp2. The two bonds are made by overlap of carbon sp2 and oxygen sp2 for the sigma bond and carbon 2p and oxygen 2p overlap for the pi bond. 175 Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals 9.6 The electron-pair geometry and molecular geometry are trigonal planar at the central carbon; the central carbon is sp2 hybridized. The sigma bond is formed between a carbon sp2 orbital and an oxygen sp2 orbital. The pi bond is formed between a carbon 2p orbital and an oxygen 2p orbital. 9.7 9.8 9.9 (a) BBr3 trigonal planar trigonal planar sp2 (b) CO2 linear linear sp (c) CH2Cl2 tetrahedral tetrahedral sp3 (d) CO32– trigonal planar trigonal planar sp2 (a) CSe2 linear linear sp (b) SO2 trigonal planar bent sp2 (c) CH2O trigonal planar trigonal planar sp2 (d) NH4+ tetrahedral tetrahedral sp3 (a) C: sp3 O: sp3 (b) From left to right: sp3, sp2, sp2 (c) N: sp3 9.10 CH2: sp3 underlined atom (a) (b) hybrid orbital set both N atoms are sp3 hybridized N C sp2 C of CH3 sp3 C of C=C and C=O (c) sp2 C of CN sp F F Si F electron-pair geometry molecular geometry hybridization octahedral octahedral sp3d 2 2– F 176 both C atoms are sp2 hybridized C of C=C 9.11 (a) CO2H: sp2 F F Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals F F (b) Se trigonal bipyramidal see-saw sp3d trigonal bipyramidal linear sp3d octahedral square planar sp3d 2 electron-pair geometry molecular geometry hybridization octahedral square pyramid sp3d 2 trigonal bipyramidal trigonal bipyramidal sp3d octahedral square pyramid sp3d 2 trigonal bipyramidal linear sp3d F F – (c) Cl I Cl F F Xe (d) F F 9.12 O F F Xe (a) F F F F O S (b) F F F F F Br (c) F F – Br Br Br (d) F 9.13 H – F P O P O O O F F tetrahedral tetrahedral sp3 sp3 177 Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals F 9.14 H S O S O O tetrahedral 9.15 O O O sp – F tetrahedral 3 sp3 The C atom is sp2 hybridized. Two of the sp2 hybrid orbitals are used to form C—Cl bonds. The third is used to form the C—O bond. The p orbital not used in the C atom hybrid orbitals is used to form the CO bond. 9.16 The C atom is sp2 hybridized. Each carbon uses two sp2 orbitals to form C-C sigma bonds with a sp2 orbital of each adjacent carbon atom and a sp2 orbital to form a C-H sigma bond with a 1s orbital of a hydrogen atom. Each carbon uses a 2p orbital to form a pi bond with another carbon 2p orbital. 9.17 The electron-pair geometry is tetrahedral, and molecular geometry is trigonal-pyramidal. The hybridization of the sulfur is sp3. 9.18 The electron-pair geometry and molecular geometry are tetrahedral. The hybridization of the sulfur is sp3. H 9.19 (a) Cl H C (b) C H3C C cis isomer 9.20 (a) H3C CH2CH3 cis isomer (b) cis and trans isomers not possible 178 H (c) C CH3 trans isomer H C C H CH3 H H CH2OH C Cl C H trans isomer Chapter 9 9.21 9.22 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals H2+: (1s)1 Bond order = 1/2(1 – 0) = 1/2 weaker H—H bond H 2: (1s)2 Bond order = 1/2(2 – 0) = 1 stronger H—H bond Li2+: (1s)2(*1s)2(2s)1(*2s)0 Bond order = 1/2(3 – 2) = 1/2 Li2– (1s)2(*1s)2(2s)2(*2s)1 Bond order = 1/2(4 – 3) = 1/2 Li2 (1s)2(*1s)2(2s)2(*2s)0 Bond order = 1/2(4 – 2) = 1 The bond order of Li2 is greater than that of either of its ions. 9.23 2p π*2p 2p 2p *2s 2s The C22– ion has a bond order of 1/2(8 – 2) = 3 (one bond and two bonds). The C2 molecule has two fewer electrons and a bond order of 1/2(6 – 2) = 2. The C22– ion is diamagnetic. 9.24 2p π*2p 2p 2p *2s 2s 1and 1 ½ bonds 2 ½ bonds total Bond order is increased ½. Yes, it is paramagnetic. 9.25 (1s)2(*1s)2(2s)2(*2s)2 (2p)2(2p)4(2p)4 (a) diamagnetic vs. paramagnetic O2 (b) 1 bond vs. 1 and 1 in O2 (c) 1 vs. 2 in O2 (d) longer bond than in O2 179 Chapter 9 9.26 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals (1s)2(*1s)2(2s)2(*2s)2 (2p)2(2p)4(2p)3 (a) paramagnetic but 1 unpaired e- vs. 2 in O2 (b) 1 and ½ vs. 1 and 1 in O2 (c) 1 1/2 vs. 2 in O2 (d) longer bond than in O2 9.27 N2 (triple) < O2 (double) < C2 (double) < B2 (single) < Li2 (single) 9.28 (a) CN-, CO, NO+, C22(b) O2-, O2, NO (c) O2-, NO 9.29 (a) ClO has 13 valence electrons. [core electrons](s)2(*s)2(p)4(p)2(p)3 (b) The HOMO is p (c) diamagnetic (d) Bond order = 1/2(8 – 5) = 3/2 9.30 One bond and 0.5 bonds (a) The NO+ ion has an even number of valence electrons (10 electrons) and so is predicted to be diamagnetic. (b) NO+: [core electrons](2s)2(*2s)2(2p)4(2p)2 The HOMO is 2p (c) Bond order = 1/2(8 – 2) = 3 (d) The bond order of NO is 2.5, whereas that of NO+ is 3. Therefore, NO+ has a stronger bond than NO. – F 9.31 F Al F F The electron-pair and molecular geometries are tetrahedral. The Al atom is sp3 hybridized. Each of these orbitals overlaps a fluorine 2p orbital to form four Al—F sigma bonds. Each F atom has a formal charge of zero and the Al atom carries a –1 formal charge. This is not a reasonable charge distribution because the less electronegative element, Al, carries a negative formal charge. 180 Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals F 9.32 F Cl F The electron-pair geometry is trigonal bipyramidal, and the molecular geometry is T-shaped. The Cl atom is sp3d hybridized. Three of these hybrid orbitals each overlap a fluorine 2p orbital to form three Cl—F sigma bonds. 9.33 (a) SO2 120º sp2 (b) SO3 120º sp2 (c) SO32– 109º sp3 (d) SO42– 109º sp3 SO2 and SO3 have the same bond angle and the S atom in each uses the same hybrid orbitals. SO 32– and SO42– have the same bond angle and the S atom in each uses the same hybrid orbitals. 9.35 – + 9.34 F Cl F F Cl F electron-pair geometry tetrahedral trigonal bipyramidal molecular geometry bent linear Cl atom hybridization sp3 sp3d CFS angle ~109.5o 180o O N O – O N O – electron-pair geometry: trigonal planar molecular geometry: bent O—N—O bond angle = 120º Average bond order = 3/2 N atom hybridization: sp2 – O 9.36 O N O – O O N O – O O N O The N atom hybridization is the same in each structure (sp2). The three sp2 hybrid orbitals are used to form N—O bonds. The p orbital not used in the N atom hybrid orbitals is used to form the NO bond. 181 Chapter 9 9.37 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals N N O N N O N N O In each structure the central N atom is sp hybridized. The other N atom hybridization changes from sp to sp2 to sp3. The two sp hybrid orbitals on the central N atom are used to form N—N and N—O bonds. The two p orbitals not used in the N atom hybridization are used to form NN and NO bonds. 9.38 9.39 O—C—O bond angle CO bond order C atom hybridization CO2 180º 2 sp CO32– 120º 4 sp2 /3 (a) All three molecules have the same molecular formula, C2H4O. They are isomers. (b) Both carbon atoms in ethylene oxide are sp3 hybridized. The CH3 carbon in acetaldehyde is sp3 hybridized and the C=O carbon is sp2 hybridized. Both carbon atoms in vinyl alcohol are sp2 hybridized. (c) H—C—H angle ethylene oxide 109º acetaldehyde 109º vinyl alcohol 120º (d) All three molecules are polar. (e) Vinyl alcohol has the strongest carbon-carbon bond, and acetaldehyde has the strongest carbon-oxygen bond. 9.40 (a) carbon 1: sp2 carbon 2: sp2 (b) angle A = 120º; angle B = 120º; angle C = 120º (c) No, cis-trans isomerism is not possible 9.41 (a) CH3 carbon atom: sp3 C=N carbon atom: sp2 N atom: sp2 (b) C—N—O angle = 120º 9.42 (a) angle A = 120º; angle B = 109º; angle C = 109º; angle D = 120º (b) carbon 1: sp2; carbon 2: sp2; carbon 3: sp3 9.43 (a) C(1) = sp2; O(2) = sp3; N(3) = sp3; C(4) = sp3; P(5) = sp3 (b) angle A = 120º; angle B = 109º; angle C = 109º; angle D = 109º (c) The P—O and O—H bonds are most polar ( = 1.3) 9.44 (a) 1 bond and 11 bonds. (b) C(1) = sp3, C(2) = sp2, O(3) = sp3 (c) The C=O bond is the shortest and strongest CO bond. (d) angle A = 109º, angle B = 109º, angle C = 120º 182 Chapter 9 9.45 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals (a) The C=O bond is the most polar bond in the molecule. (b) There are 18 bonds and 5 bonds in the molecule. (c) The trans isomer is shown. The cis isomer is H H H C H C C C H C C C C H C O H H (d) All carbon atoms in the molecule are sp2 hybridized. (e) All three angles are 120º. – O 3– O O I 9.46 O O O O O O electron-pair geometry tetrahedral trigonal bipyramidal molecular geometry tetrahedral trigonal bipyramidal hybridization of I atom 9.47 I sp 3 sp3d (a) sp3d in SbF5, sp3d 2 in SbF6– (b) H F H + The geometry of H2F+ is bent, and the F atom is sp3 hybridized. O Xe 9.48 O O O Xe O O O Electron-pair geometry tetrahedral tetrahedral Molecular geometry trigonal pyramidal tetrahedral 3 Xe sp hybridized sp3 hybridized 2-– 9.49 (a) O O bond order = 1 (b) [core electrons](2s)2(*2s)2(2p)4(2p)2(π*2p)4 bond order = 1/2(8 – 6) = 1 (c) Yes, the two bonding theories lead to the same magnetic character (diamagnetic) and bond order. 183 Chapter 9 9.50 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals N2 [core electrons](2s)2(*2s)2(2p)4(2p)2 N2+ [core electrons](2s)2(*2s)2(2p)4(2p)1 N2– [core electrons](2s)2(*2s)2(2p)4(2p)2(π*2p)1 N2 N2+ N 2– (a) diamagnetic paramagnetic paramagnetic (b) 2 bonds 2 bonds 1 1⁄2 bonds 3 2 1/2 2 1/2 (c) bond order N2 < N2+ ≈ N2– (d) —increasing bond length N2+ ≈ N2– < N2 (e) —increasing bond strength 9.51 B2 and O2 are paramagnetic, Li2, B2, and F2 have a bond order of 1, C2 and O2 have a bond order of 2, and N2 has the highest bond order, 3. 9.52 Molecule or ion HOMO paramagnetic π* – (b) OF diamagnetic π* (c) O22– diamagnetic π* (d) Ne2+ paramagnetic *2p (e) CN paramagnetic (a) NO 9.53 Magnetic behavior CN 2 2 2p 4 [core electrons](2s) (*2s) (2p) (2p) 1 (a) The HOMO is 2p (b) Bond order = 1/2(7 – 2) = 2 1/2 (c) One-half net bond and two net bonds (d) paramagnetic 9.54 (a) C6 ring carbon atoms: sp2; side chain carbon atoms: sp3; N atom: sp3 (b) angle A = 120º; angle B = 109º; angle C = 109º (c) 23 bonds and 3 bonds (d) The molecule is polar (e) The H+ ion attaches to the most electronegative atom in the molecule, N, and this is confirmed by the electrostatic potential map (most negative region near N). 9.55 (a) All of the C atoms are sp3 hybridized (b) C—O—H angle = 109º (c) The molecule is polar (d) The six-member ring is non-planar. The ring could only be planar if the carbon atoms were sp2 hybridized as in benzene. 184 Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals F F B 9.56 F F H H C C is sp2 hybridized, H—C—C = 120º C H H B is sp2 hybridized, F—B—B = 120º B H H N N is sp3 hybridized, H—N—N = 109º N H H H O O is sp3 hybridized, H—O—O = 109º O H 9.57 (a) The geometry about the boron atom is trigonal planar in BF3, tetrahedral in H3N—BF3. (b) Boron is sp2 hybridized in BF3, sp3 hybridized in H3N—BF3. (c) Yes (d) The ammonia molecule is polar with the N atom partially negative. While the BF 3 molecule is nonpolar overall, each of the B–F bonds is polarized such that the B has a partial positive charge. The partially negative N in NH3 is attracted to the partially positive B in BF3. (e) One of the lone pairs on the oxygen in H2O can form a coordinate covalent bond with the B in BF3. 9.58 (a) Even though the atoms are sp3 hybridized, the bond angles in the three-member ring must be 60º. (b) sp3 (c) Because the angles are significantly less than the expected value of 109º, the ring structure is strained and relatively easy to break. (The molecule is reactive.) (d) Yes, the molecule is polar. The negative partial charge is near oxygen and the hydrogen atoms carry a partial positive charge. 9.59 (a) NH2–: electron pair geometry, SO3: electron pair geometry, trigonal planar; tetrahedral; molecular geometry, bent; sp3 molecular geometry trigonal planar; sp2 O N H S H O O 185 Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals – O H (b) H S O The angles around N and S are approximately 109º. N O (c) The hybridization of the N atom does not change (sp3 in NH2– and H2N—SO3–). The S atom hybridization changes from sp2 in SO3 to sp3 in H2N—SO3–. (d) SO3 is an electron pair acceptor. The S atom carries a positive partial charge so it is predicted to act as an electron-acceptor. 9.60 (a) The keto and enol forms are not resonance structures because both electron pairs and atoms have been rearranged. (b) The terminal —CH3 carbon atoms are sp3 hybridized and the three central carbon atoms are sp2 hybridized in the enol form. In the keto form, the terminal —CH3 carbon atoms and the central C atom are sp3 hybridized and the two C=O carbon atoms are sp2 hybridized. (c) Enol form: Keto form: —CH3 carbon atoms tetrahedral central three carbon atoms trigonal planar —CH3 carbon atoms tetrahedral central —CH2— carbon atom tetrahedral C=O carbon atoms trigonal planar Only the center carbon atom changes geometry, from trigonal planar to tetrahedral – H (d) CH3 C C O C CH3 O – H CH3 C C C O CH3 O – H CH3 C O C C CH3 O (e) Possible for the enol form (f) Yes. The negative partial charges are on the oxygen atoms. The positive partial charges are on the hydrogen atoms, especially the O—H hydrogen. 186 Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals 9.61 Hybridization of the carboxylate C is sp2. C-C bond: overlap of sp2 hybrid orbital on the carbon of the carboxylate with sp3 hybrid orbital on the methyl carbon. C-O single bond: The Lewis structure by itself would make it appear that this bond is formed by the overlap of an sp2 hybrid orbital on carbon with an sp3 hybrid orbital on the oxygen. In the resonance hybrid, however, the oxygen would also have sp2 hybridization. 9.62 (a) The central atoms are sp hybridized in all four molecules/ions. The most stable resonance structures are shown below (i.e, those that minimize formal charges and place negative charges on the most electronegative atom). For the C of CO2 and the central nitrogen of N3-, both bonds are made from overlap of an sp hybrid orbital on the central atom with an sp2 hybrid orbital from an outer atom. Both bonds are made from overlap of a 2p orbital on the central atom with a 2p orbital on an outer atom. For the C of OCN- and the central nitrogen of N2O, the bond comprising the single bond is made from an sp hybrid orbital on the central atom and an sp3 hybrid orbital on the outer atom. The bond of the triple bond is made from an sp hybrid orbital on the central atom and a sp hybrid orbital on the outer atom. The bonds of the triple bonds are made from overlap of 2p orbitals on the central atom and 2p orbitals of the outer atom. (b) For each of the four molecules are ions, three resonance structures can be drawn – one with two double bonds, one with a triple bond on the left and a single bond on the right, and one with a triple bond on the right and a single bond on the left. The structure that minimizes formal charges, places opposite formal charges on adjacent atoms, places like formal charges as far apart as possible, and places a negative formal charge on the most electronegative atom or a positive formal charge on the least electronegative atom is the resonance structure that should contribute the most to the actual structure. For CO 2, the formal charge on each atom in the structure in part (a) is zero; the bond lengths are expected to be equivalent and to be typical of a carbon-oxygen double bond. For N3-, the formal charge on each outer atom is -1 and on the central atom is +1in the structure in part (a); the bond lengths are expected to be equivalent and to be typical of a nitrogen-nitrogen double bond. For OCN-, the formal charges are oxygen, -1; carbon, 0; and nitrogen, 0. The carbon-oxygen bond should be close to single bond length, and the carbon-nitrogen bond should be close to triple bond length. For N2O, the central nitrogen has a formal charge of +1, the outer 187 Chapter 9 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals nitrogen has a formal charge of 0, and the oxygen has a formal charge of -1. The nitrogen-nitrogen bond should approach triple bond length, while the N-O bond should approach a single bond length. 9.63 MO theory pictures one net bond for each S-O linkage plus a contribution from bonding. The bonding in this molecule will be similar to that in O3 discussed in the text. There will be two electrons in a bonding MO and two electrons in a non-bonding MO. This gives an overall bond order of 1 for the bonding in the entire molecule and there a net bond order of 0.5 for each S-O linkage. The total bond order for each S-O linkage is therefore 1.5 (1 from bonding and 0.5 from bonding). 9.64 The electronic geometry at the nitrogen atoms is trigonal planar, and the molecular geometry is bent. The nitrogen atoms are sp2 hybridized. The N=N double bond is comprised of a bond (made from the overlap of sp2 hybrid orbits on the nitrogen atoms) and a p bond (made from overlap of 2p orbitals on the nitrogen atoms). 9.65 The maximum number of hybrid orbitals that a carbon atom can form is four and the minimum number that can be formed is two. Carbon has only four valence orbitals, one s and three p, so it cannot form more than four hybrid orbitals. 9.66 (a) CF4 is isoelectronic with BF4– (32 valence electrons) (b) SiF4 (32 valence electrons) and SF4 (34 valence electrons) are not isoelectronic (c) BF4–: sp3 9.67 SiF4: sp3 SF4: sp3d (a) C atom: sp2; N atom: sp3 (b) Another resonance structure (showing only the peptide linkage and the formal charges on O and N) is This resonance structure is less important owing to the separation of charges. (c) O C –1 +1 N H The fact that the amide link is planar indicates that the resonance structure shown above has some importance. The highly electronegative oxygen (C=O) and nitrogen (–NH2 group) atoms carry partial negative charges and the –O–H and amide N–H hydrogen atoms carry partial positive charges 188 Chapter 9 9.68 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals The higher the bond order, the shorter the bond length and the larger the bond energy. Acetylene has a carbon-carbon triple bond (bond order = 3), so it has the shortest and strongest carbon-carbon bond. Ethane has a carbon-carbon single bond (bond order = 1), so it has the longest and weakest carbon-carbon bond. 9.69 Molecular orbital theory correctly predicts the electronic structures for odd-electron molecules and other molecules such as O2 that do not follow the electron-pairing assumptions of the Lewis dot structure approach. 9.70 Valence bond theory uses resonance to explain the bond order of 1.5 in O 3. Molecular orbital theory uses three molecular orbitals (bonding, nonbonding, and antibonding) combined with sigma bonding and antibonding molecular orbitals to explain the bond order. 9.71 Orbital C < Orbital B < Orbital A 9.72 (a) The number of hybrid orbitals is always equal to the number of atomic orbitals used. (b) No. All hybrid orbital sets involve an s orbital. (c) The energy of the hybrid orbital set is the weighted average of the energy of the combining atomic orbitals. 9.73 B surrounded by three electron pairs: electron-pair geometry and molecular geometry are both trigonal planar; the hybridization is sp2; formal charge is zero. B surrounded by four electron pairs: electron-pair geometry and molecular geometry are both tetrahedral; the hybridization is sp3; the formal charge is -1. 9.74 (a) two parallel p orbitals on the first C and the central carbon overlap to form a pi bond. The last C must be rotated so that the p orbitals for it and the central C align to form another pi bond. (b) C(1) sp2; C(2) sp; C(3) sp2 (c) The overlaps of C1 and C3 atoms’ sp2 orbitals with the C2 atom’s sp orbitals form the sigma bonds. The overlap of the carbon’s 2p orbitals forms the pi bonds. 9.75 HF has a bond order of 1. HF2- has a bond order of 1 for the entire three-center-four-electron bond and thus a 0.5 bond order per H-F linkage. It should therefore be easier to break the bond in HF 2-, leading to a smaller bond enthalpy for HF2- than for HF. 9.76 (a) Hybridization is sp3d. The Xe-F bonds are formed by overlap of an sp3d hybrid orbit on Xe with an sp3 hybrid orbital on F. The lone pairs on Xe are in sp3d hybrid orbitals. (b) Along the z-axis, bonding, non-bonding, and antibonding orbitals can be constructed from combinations of the 5pz orbital on the Xe and the two 2pz orbitals on the fluorines. Four electrons are placed in the bonding and nonbonding orbitals. 189 Chapter 9 9.77 Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals (a) Br 0.4569 g/(79.904 g/mol) = 0.005718 mol Br P 0.5431 g F/(18.9984 g/mol) = 0.02859 mol F 0.02859 mol F/0.005718 mol Br = 5.000 mol F: 1 mol Br Empirical formula is BrF 5 (b) Molecular geometry is square pyramidal; hybridization is sp3d2. (c) Yes, the square pyramidal structure is expected to be polar. 9.78 (a) A resonance structure can be drawn with double bonds between the alternate carbons and nitrogens of the ring; the average of the two resonance structures would result in identical bonds with a bond order of 1.5 between each C and N of the ring. (b) H = [(1 mol)(fHo(melamine(s)) + (6 mol)(fHo(NH3(g))) + (3mol)(fHo(CO2(g)))] – (6 mol)(fHo(urea(s))) = [(1 mol)(-66.1 kJ/mol) + (6 mol)(-45.90 kJ/mol) + (3 mol)(-393.509 kJ/mol)] – (6 mol)(-333.1 kJ/mol) = +477 kJ 9.79 (a) Br 0.9090 g/(79.904 g/mol) = 0.01138 mol Br 0.0910 g/(15.9994 g/mol) = 0.00569 mol O 0.01138 mol Br/0.00569 mol O = 2.00 mol Br: 1 mol O Empirical formula OBr 2 The oxygen should be sp3 hybridized. (b) 13 valence electrons; (s)2(*s)2(p)4(p)2(*p)3; HOMO is *p SOLUTIONS TO APPLYING CHEMICAL PRINCIPLES: PROBING MOLECULES WITH PHOTOELECTRON SPECTROSCOPY 1. metal 2. E = h = hc/ = (6.626 x 10-34 J·s)(2.998 x 108 m/s)/(58.4 x 10-9 m) = 3.40 x 10-18 J/photon (3.40 x 10-18 J/photon)(6.022 x 1023 photons/mol) = 2.05 x 106 J/mol = 2.05 x 103 kJ/mol 3. 2p 4. h3.40 x 10-18 J from problem 2 above IE = h3.40 x 10-18 J – 4.23 x 10-19J = 2.98 x 10-18 J/electron (2.98 x 10-18 J/electron)(6.022 x 1023 electron/mol)(1 kJ/1000 J) = 1.79 x 10 3 kJ/mol (2.98 x 10-18 J)(1 eV/1.60218 x 10-19 J) = 18.6 eV 190 Chapter 9 5. Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals The 15.6 eV and 16.7 eV ejected electrons came from bonding orbitals. Removing an electron from a bonding orbital weakens the bonds and thus results in a longer bond length. The 18.6 eV ejected electron comes from an antibonding orbital. 191