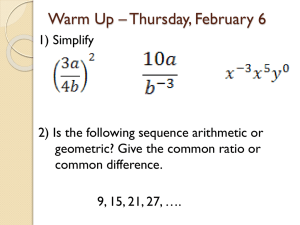Balancing Nuclear Equations and Half-life Calculations
advertisement

Balancing Nuclear Equations and Half-life Calculations Determine “X” in reactions 1-5 below. 1. 212 84 Po 82208Pb + X 2. Cs-137 Ba-137 + X 3. As-78 - + X 4. 4 9 Be + C-12 + X 5. K-38 Ar-38 + X Half-life 6. The half-life of plutonium-239 is 24,110 years. If you have an original mass of 100 g, how much remains after 96,440 years? 7. The half-life of polonium-218 is 3.0 minutes. If you start with 16 tons, how long will it be before only 1 ton is left? 8. The half-life of thorium-227 is 18.72 days. How many days are required for three-fourths of a given amount to decay? 9. Gold-198 has a half-life of 2.7 days. If you have an original mass of 50 g, how much will remain after 1 week? 10. Technetium-99 has a half-life of 6.0 hours. If an original mass contains 100 mg, how much will remain after 2.0 days? 11. Calculate the half-life of an element having an original mass of 276 g and a final mass of 4.76 g after decaying for 4.39 days.