doc
advertisement
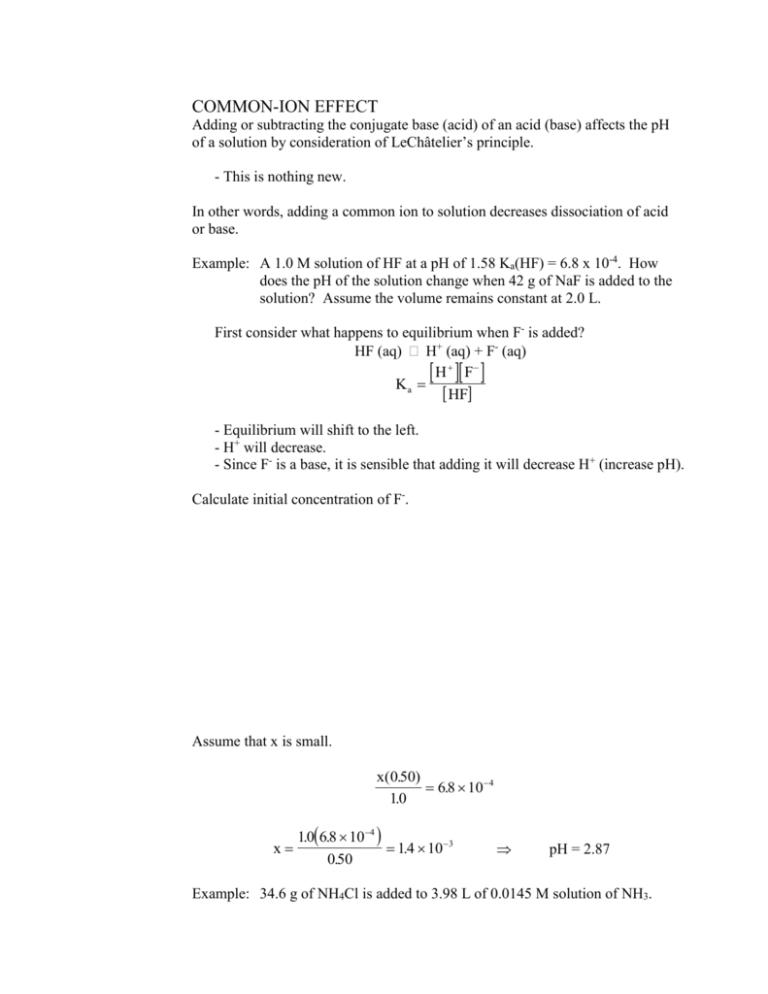
COMMON-ION EFFECT Adding or subtracting the conjugate base (acid) of an acid (base) affects the pH of a solution by consideration of LeChâtelier’s principle. - This is nothing new. In other words, adding a common ion to solution decreases dissociation of acid or base. Example: A 1.0 M solution of HF at a pH of 1.58 Ka(HF) = 6.8 x 10-4. How does the pH of the solution change when 42 g of NaF is added to the solution? Assume the volume remains constant at 2.0 L. First consider what happens to equilibrium when F- is added? HF (aq) H+ (aq) + F- (aq) H F Ka HF - Equilibrium will shift to the left. - H+ will decrease. - Since F- is a base, it is sensible that adding it will decrease H+ (increase pH). Calculate initial concentration of F-. Assume that x is small. x(0.50) 6.8 10 4 10 . 10 . 6.8 104 x 14 . 10 3 0.50 pH = 2.87 Example: 34.6 g of NH4Cl is added to 3.98 L of 0.0145 M solution of NH3. Kb(NH3) = 1.8 x 10-5. a) What is the pH of the original solution before the addition of the NH4Cl? Assume that x is small. x2 18 . 105 0.0145 x2 0.014518 . 105 2.6 107 x = 5.1 10-4 pOH = 3.29 pH = 10.71 b) Calculate the pH of the solution after the addition of the NH4Cl. Assume volume remains constant. Calculate initial concentration of NH4+. 34.6 g 1 mol . 0162 . M 53.4912 g 3.98 L NH4+ (aq) H+ (aq) + NH3 (aq) H NH Ka NH 4 3 Assume that x is small. x 0.0145 5.6 1010 (0162 . ) 0162 . 5.6 1010 x 6.26 10 9 pH = 8.20 0.0145 BUFFERS - Buffers are solutions of conjugate acid/base pairs. - Buffers change pH slowly when acid or base is added. - Buffers take advantage of common-ion effect Consider solution of 2.0 M HC2H3O2 and 1.0 M NaC2H3O2. HC2H3O2 (aq) H+ (aq) + C2H3O2- (aq) - Because of common-ion effect, acid will dissociate very little. When a strong acid is added to the solution, it neutralizes the base, C2H3O2-. HCl (aq) + C2H3O2- (aq) HC2H3O2 (aq) + Cl- (aq) When a strong base is added to the solution, it neutralizes the acid, HC2H3O2. NaOH (aq) + HC2H3O2 (aq) NaC2H3O2 (aq) + H2O (l) What happens to the solution if we add enough HCl to change to acetate concentration from 1.0 to 0.9? - Base is converted to acid. Therefore, acetic acid concentration must change from 2.0 to 2.1. before neutralization 0.1 M 1.0 M 2.0 M 0.0 M HCl (aq) + C2H3O2- (aq) HC2H3O2 (aq) + Cl- (aq) 0.0 M 0.9 M 2.1 M 0.1 M after neutralization - Now calculate pH Ka H C2 H3O2 HC2 H3O2 x(0.9 x) x(0.9) 1.8 105 2.1 x 2.1 x 4.2 105 pH = 4.38 Compare this pH to pH of 0.1 M HCl solution pH = 1.0 **The acetic acid/acetate solution has “buffered” the effects of addition of acid.** Buffer Capacity - Amount of acid or base that can be added to a buffer solution before its pH changes appreciably. Consider two 1.0 L ammonia/ammonium buffer solutions. Buffer 1 1.0 M NH3 1.0 M NH4+ H NH 3 NH 4 K 10. 5.6 1010 Ka H K a NH 4 NH 3 a 10. pH = 9.25 What happens when 0.1 mol of NaOH is added? 0.1 M 1.0 M 1.0 M + NaOH (aq) + NH4 (aq) NH3 (aq) + H2O (l) 0.9 M 0.0 M H Ka NH K NH 4 3 a 1.1 M 0.9 4.6 10 10 11 . pH = 9.34 - High concentration of conjugate acid/base pairs make for high buffer capacity. Buffer 2 0.01 M NH4+ 0.01 M NH3 H NH Ka NH 3 4 H K NH K NH 4 a a 3 0.01 5.6 10 10 0.01 pH = 9.25 What happens when 0.1 mol of NaOH is added? 0.1 M 0.01 M 0.01 M + NaOH (aq) + NH4 (aq) NH3 (aq) + H2O (l) 0.09 M 0.02 M 0M - pH is dictated by excess of strong base. pOH = -log(0.09) = 1.05 pH = 12.95 - Buffer 1 has much more capacity than Buffer 2. - Capacity of buffer depends on concentration of conjugate acid/base pairs. - high concentrations high capacity - low concentrations low capacity Henderson-Hasselbalch equation - Relates pH of buffer solution to concentrations of acids and bases. - No substantive difference from Ka expression, just in a more convenient form. Derivation Recall rules of logarithms log ab log a log b H A Ka a log b log b a HA H K HA A a HA log H log K a A HA HA pH log K a log pK a log A A HA pH pK a log A 1 A pK a log HA [ base] pH pK a log [acid ] Observations about the Henderson-Hasselbalch equation - pH of buffer is approximately pKa of acid - exactly equal when [base] = [acid] - Can get same answers from ICE table since x is small. - Equation neglects changes in acid or base when strong acid or base is added. - Equation is used to monitor pH of weak acid solution when titrated with a strong base. - When choosing buffer system for a particular pH, choose acid such that its pKa pH Example: What is the pH of a solution with 1.0 M NaC2H3O2 and 2.0 M HC2H3O2? Ka(HC2H3O2) = 1.8 10-5 Note that answer agrees with answer to same problem done previously with ICE table. Example: How many grams of NaH2PO4 and Na2HPO4 are needed to make 2.4 L of a buffer with a pH of 7.40? The concentrations of the buffer species should approximately 0.1 M. Ka (H3PO4) = 6.2 10-8 = Ka(H2PO4-). 2 H2PO4- (aq) H+ (aq) + HPO42- (aq) [ base] pH pK a log [acid ] [ base] log . 7.40 7.21 019 [acid ] [ base] 7.40 7.21 log [acid ] [ base] 100.19 15 . [acid ] Since the pH of the buffer is dependent only on the ratio of acid to base, let us allow the concentration of the acid to be 0.10 M and the concentration of the base to equal to 0.15 M Grams of acid, NaH2PO4 2.4 L 010 . mol 119.98 g 28.8 g L mol Grams of base, Na2HPO4 2.4 L 015 . mol 14196 . g 511 . g L mol The pH of blood serum is 7.40; thus, phosphate buffers are used in many intravenous formulations. ACID-BASE TITRATIONS - We can measure amount of acid (base) in a solution by adding an equal amount of base (acid). - equivalence point – where amount of acid equals amount of base **moles of acid = moles of base** - How do we know when moles of acid = moles of base? Use indicator. - indicator – substance which changes color when pH is changed. indicator methyl red litmus bromothymol blue phenolphthalein pH of color change 5.0 6.5 7.1 9.4 acidic color red red yellow colorless basic color yellow blue blue pink - endpoint – where indicator changes color. - When doing titration, choose indicator whose endpoint is at approximately the expected equivalence point. Titration procedures Deadstop titration - A precise amount of acid (base) is measured with a pipet. - Class A pipet tolerance = 0.001 mL - Indicator is added. - For titration of strong acid with strong base, phenolphthalein is often used, but bromothymol blue is more accurate with experienced eyes. - A precise amount of base (acid) is added slowly using buret until indicator changes. Potentiometric titration - In a titration, a precise amount of acid (base) is measured with pipet. - pH of acid (base) is monitored with a pH meter as base is added. - Data of pH versus volume of base (acid) added is recorded and plotted to yield titration curve. Titration curves - A titration curve is a plot of pH versus volume of base (acid) added. - Curve yields equivalence point and pKa (pKb) of acid (base) titrated. Strong acid – strong base titration curve - Curve is a very sharp S shape. - Equivalence point occurs at pH = 7 - moles of acid = moles of base - nH+ = nOH- Near equivalence point, pH changes over 6 units. - drastic change allows flexibility with choice of indicator. - Note small slope at beginning and end of curve - pH changes very little until equivalence point is reached. - For strong base titrated with strong acid, curve is reversed. Titration of 50.00 mL of Strong Base with 0.1000 M HCl 14 13 12 11 10 9 pH 8 7 6 5 4 3 2 1 0 0 25 50 75 volum e of HCl (m L) 100 125 Weak acid – strong base titration curve - Curve is less sharp - pKa is found at point halfway between starting point and equivalence point. - At halfway point, half of acid is converted to conjugate base, thus [HA] = [A-], thus pH = pKa. [ base] pH pK a log pK a log(1) pK a [acid ] - The halfway point is where a buffer solution has been created. - This halfway point is called an inflection point. - At equivalence point, change in pH is not as dramatic as strong acid – strong base titration. - Need to be more careful about choosing indicator - Slope at beginning and end of curve are slightly steeper. - Indicates that H+ is reacting to smaller extent with A- than with OH-. - The stronger the conjugate base, the steeper the slope. - For weak base titrated with strong acid, curve is reversed with pKa at halfway point between start and equivalence point. - recall that pKb = pKw – pKa - pH at equivalence point not necessarily 7. - We have four regions where can calculate pH during a weak acid-strong base titration. i) Initial (calculate the pH of the weak acid) ii) Buffer region (Henderson-Hasselbalch) iii) Equivalence point (weak base solution) iv) Excess titrant (calculate excess OH- conc.) Example: Calculate the pH of the resultant solution after 25.00 mL of 0.1102 M HF is titrated with the following volumes of 0.1004 M NaOH. a) 0.00 mL This is a weak acid solution. b) 15.63 mL Now we need to know where this point is on the titration curve. Compare the amount of acid to the amount of base. The amount of base is less than the amount of acid; therefore, this point is in the buffer region. Before considering the equilibrium, consider the acid-base reaction first. HF (aq) + OH- (aq) H2O (l) + F- (aq) After the chemical reaction: n F 1.569 mmol n HF remaining n HF total n HF used n HF total n OH used 2.755 mmol 1.569 mmol 1.186 mmol [ base] pH pK a log [acid ] 1.569 mmol 40.63 mL pH log 6.8 10 4 log 1.186 mmol 40.63 mL 1.569 mmol pH 3.17 log 3.17 0.12 3.29 1.186 mmol c) 25.00 mL Where on the titration curve is this point? Still in the buffer region (barely!) After the chemical reaction: n F 2.510 mmol d) 27.44 mL We’re at the equivalence point! We now have a solution of sodium fluoride (a weak base). F- (aq) + H2O (aq) HF (aq) + OH- (aq) What is the fluoride concentration? [ F ] moles of F 25.00 mL 01102 . M 0.05254 M total volume 25.00 mL 27.44 mL Now we can treat solution as a weak base. Assume x is small. x2 15 . 1011 x 2 0.0525415 . 1011 0.05254 x = 8.8 10-7 = [OH-] pOH = -log 8.8 10-7 = 6.06 pH = 14.00 – pOH = 7.94 If we are doing a deadstop titration, phenolphthalein (pKa = 9.4) would not be an optimal indicator. We would get a better result with phenol red instead. (pKa = 7.9) e) 30.34 mL This point is in the excess titrant region. After the chemical reaction: n F 2.755 mmol n OH remaining n OH total n OH used 3.046 mmol 2.755 mmol 0.291mmol [OH ] moles of OH 0.291 mmol 0.00526 M total volume 55.34 mL pOH log[OH ] log 0.00526 2.28 pH 14.00 pOH 14.00 2.28 11.72 Polyprotic acids Each proton of polyprotic acid has an equivalence point and a buffer region to find pKa. Titration of 25.00 mL of Diprotic Acid with 0.1000 M NaOH 13 12 11 10 pKa 2 9 8 equivalence points pH 7 6 5 4 3 2 pKa 1 1 0 0 25 50 75 100 125 150 volum e of NaOH (m L) Note: pKa points and equivalence points are at inflection points SOLUBILITY EQUILIBRIA An untruth: Solid substances are either soluble or insoluble in solvent. The truth: All ionic compounds are soluble though some have extremely small solubilities. The less soluble substances are in equilibrium with their dissociated ions. MX (s) M+ (aq) + X- (aq) M X Kc K sp M X MX Ksp – solubility product constant In general for MAXB (s) A Ma+ (aq) + B Xb- (aq) Ksp M a X b A B Examples: Ca(OH)2 Ag3AsO4 SrCO3 **Ksp tells us concentration of ions in a saturated solution.** Example: Calculate pH of Ca(OH)2 solution. Ksp = 5.5 10-6 Ca(OH)2 (s) Ca2+ (aq) + 2 OH- (aq) Ksp Ca 2 OH 55 . 106 2 Solubility – The amount of solute (in grams or moles) that can be dissolved in a specific amount of solvent. - If solubility is given, Ksp can be calculated. - If Ksp is given, solubility can be calculated. Example: The solubility of Ca3(PO4)2 is 2.2110-5 g/100 mL Calculate Ksp. In a problem like this, consider the change in dissociation (“x” variable) to be the molar solubility. Thus we will need to convert the mass solubility to molar solubility. Keep in mind that we’ll need to convert the units in the denominator of 100 mL to L. (100 mL = 0.1 L) 2.21105 g 100 mL 1mol 7.12 107 M 100 mL 0.1L 310.18g Write Ksp expression for Ca3(PO4)2 (s) 3 Ca2+ (aq) + 2 PO43- (aq) Ksp Ca 2 PO4 3 3 2 A form of calcium phosphate, hydroxyapatite Ca10(PO4)6(OH)2, is the major mineral component of bone. Example: The Ksp of PbCl2 is 1.1710-5. Calculate its solubility in g/100 mL. PbCl2 (s) Pb2+ (aq) + 2 Cl- (aq) Remember: “x” is the molar solubility. Ksp Pb2 Cl 117 . 105 2 Ksp x 2 x 4x3 117 . 105 2 117 . 105 x 0.0143 M 4 0.0143mol 0.1L 278.11 g 0.398 g /100 mL L 100 mL mol x3 FACTORS THAT AFFECT SOLUBILITY Common ion effect - Addition of an ion in a solubility product reduces solubility. (LeChâtelier’s principle) Consider BaCrO4 (s) Ba2+ (aq) + CrO42- (aq) - Addition of BaCl2 (for example) shifts equilibrium to left, thus reducing solubility. Example: Ksp(BaCrO4) = 1.1710-10. Calculate the molar solubility of BaCrO4 a) in a solution of 0.100 M BaCl2 BaCrO4 (s) Ba2+ (aq) + CrO42- (aq) K sp Ba 2 CrO 4 2 117 . 10 10 Yikes! A quadratic equation Relax. Assume x is small Ksp 0100 . x 117 . 1010 x 117 . 109 M s While we’re at it, let’s calculate mass solubility. b) in a solution of 0.00100 M BaCl2 Initial Change Equil. [Ba2+] 0.00100 +x 0.00100 + x [CrO42-] 0 +x +x Ksp 0.00100 x 117 . 1010 x 117 . 107 M s In pure water, 1.17 107 mol 0.1L 253.32 g 2.96 106 g /100 mL L 100 mL mol s 1.08 105 M BaCrO4 is more soluble in a more dilute solution of BaCl2. pH of solution - pH affects solubility of hydroxides since [OH-] is related to [H+]. - pH affects solubility of fluorides, oxalates, phosphates, etc…, that is, salts with weak acid anions. Calculate the Cu(II) concentration in a saturated solution of Cu(OH)2. Ksp(Cu(OH)2) = 2.210-20. a) when pH = 10.00 pH = 10.00 [H+] = 1.010-10 [OH-] = 1.010-4 Ksp Cu2 OH 2.2 1020 2 Cu 2 2.2 1020 2.2 1020 OH 1.0 10 - In this case [Cu ] is also molar solubility. 2 4 2 2+ b) when pH = 5.00 pH = 5.00 [H+] = 1.010-5 [OH-] = 1.010-9 2.2 1012 M Ksp Cu2 OH 2.2 1020 2 Cu 2 2.2 1020 OH 2 2.2 1020 10. 10 9 2 2.2 102 M Example: How does pH affect solubility of SrCO3? SrCO3 (s) Sr2+(aq) + CO32- (aq) H+ (aq) + CO32- (aq) HCO3- (aq) Consider both equilibria together. SrCO3 (s) + H+ (aq) Sr2+(aq) + HCO3- (aq) Increasing pH, decreases solubility. Decreasing pH, increases solubility. True in general for solubilities of salts of weak acids. FRACTIONAL PRECIPITATION Consider the use of Q (ion product) to determine precipitation. MX (s) M+ (aq) + X- (aq) Q = [M+][X-] If Q > Ksp If Q = Ksp If Q < Ksp precipitation occurs saturated solution precipitate dissolves Example: A sample has 2.410-5 M of Pu3+ in an acidic solution. To what pH must the solution be adjusted to begin precipitation of the plutonium as Pu(OH)3? Ksp = 2.010-20 Thus, the solution will not begin to precipitate until enough base is added to adjust the pH to 8.97. When two metal ions are in solution, they can be separated from one another if their Ksp for a specific anion are very different. Example: Consider a solution of 0.114 M Pb2+ (aq) and 0.214 M Bi3+ (aq). Ksp(PbI2) = 7.110-9 Ksp(BiI3) = 8.110-19 a) What is the minimum concentration of I- needed to start precipitation of the Pb2+ ion? b) What is the minimum concentration of I- needed to start precipitation of the Bi3+ ion? c) What range of concentrations of I- can be used to precipitate only Bi3+? 1.6 10-6 M < [I-] < 2.5 10-4 M Bismuth and lead are commonly found together in ores. COORDINATION COMPLEXES Lewis acid/base theory is useful to explain structure of metal ions in aqueous solution (coordination complexes). Consider Cu2+ ion. CuSO4 (s) + very pale blue H2O (l) CuSO4 (aq) bright blue What has changed? - In the solid, Cu2+ is surrounded by SO42- In the solution, Cu2+ is surrounded by H2O - Cu2+ acts as Lewis acid. - H2O acts as Lewis base. H H O H H O O Cu2+ H H O H Consider the following equilibrium H CoCl42- + 6 H2O Co(H2O)62+ + 4 Cl- Lewis acid Lewis base H H O Cl- H H Cl- Cl- Co2+ O O Co2+ H H O ClH blue pink Ag+ + 2NH3 [Ag(NH3)2]+ Lewis acid Lewis base H H H N H H Ag+ N H H AgCl (s) + 2NH3 [Ag(NH3)2]+ + Cl- (aq) Ammonia is stronger Lewis base than chloride when silver ion is the Lewis acid. Complexation of metal ions - The addition of substances with strong Lewis base character can react with certain metal ions to form soluble complexes. - Formation of complexes increases solubility of “insoluble” salt. Consider: Silver diammine complex Ag+ + 2NH3 [Ag(NH3)2]+ Ag( NH 3 ) 2 Kf . 107 2 17 Ag NH 3 - Note that Kf (formation constant) tells us that formation of the complex is overwhelmingly favored. - The ammonia will stay bound to the silver ion until a stronger Lewis base is added to the solution. Example: How does the addition of ammonia affect the solubility of AgCl? AgCl (s) Ag+ (aq) + Cl- (aq) Ag+ (aq) + 2NH3 (aq) [Ag(NH3)2]+ (aq) AgCl (s) + 2NH3 (aq) [Ag(NH3)2]+ (aq) + Cl- (aq) Kc = Ksp Kf = (1.8 10-10) (1.7 107) = 0.00306 - From LeChâtelier’s principle, addition of ammonia increases solubility. Other substances that form complexes with metal ions I-, Br-, SCN-, Cl-, NO3-, F-, OH-, H2O, CN-, CO Amphoteric Metals Metals generally react with acids to form metal ion solutions. Ni (s) + 2H+ (aq) Ni2+ (aq) + H2 (g) However, some metals also react with bases to form metal ion solutions. Al (s) + 4 OH- (aq) Al(OH)4- (aq) Amphoteric metals react in both acid and bases to form ions. The oxides and hydroxides of amphoteric metals will dissolve in acid and base. Amphoteric metals tend to be close to the metal/nonmetal boundary. A partial list of amphoteric metals includes: Si, Al, Zn, Ga, Ge, As, Sn, Sb, Pb, etc… [Read Section 17.7 for help in lab (will not be tested in lecture).]








