Solubility Product Constant
advertisement

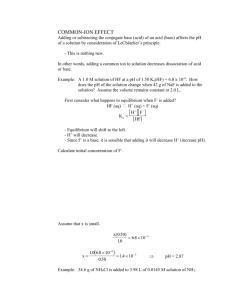
Solubility Product Constant Ksp Chapter 17 I. BaSO4(s) 2+ Ba (aq)+ 2SO4 (aq) Barium Sulfate has a certain solubility in water. At some point when enough is added the solution will become saturated, and some solid will remain. Ksp = [Ba2+] [SO42-] solubility product constant Even though the concentration the solid is omitted for the equilibrium to exist some must exist. II. Solubility and Ksp The smaller the value of Ksp the lower the solubility Solubility – the amount of a substance that dissolves when producing a saturated solution is often expressed in grams of solute /liter of solution at saturation point. Solubility product constant (Ksp) – describes the concentrations) of dissolved ions, or substances at saturated equilibrium and is a unit less expression. Solubility and Ksp Solubility of a substance may change as the concentrations of various ions change ( including H+), however the value of Ksp is unique for a given solute at a specific temperature. III. Calculating Ksp from the solubility Ex. A liter of a solution saturated at 25OC with calcium oxalate, is evaporated to dryness, giving a 0.0061 g residue. Calculate the solubility product constant for this salt. CaC2O4(s) Ca+2(aq) + C2O42-(aq) 0.0061g CaC2O4 (1 mole/128 g) = 4.8x10-5 mol 1mol CaC2O4 = 1 mol Ca+2 = 1 mol C2O42Ksp = [Ca+2] [C2O42-] = [4.8x10-5]2 = 2.3x10-9 More complicated example By experiment, it is found that 1.2 X10 -3 moles of lead II iodide dissolves in 1 liter of aqueous solution at 25oC. What is the Ksp of PbI2 at this temperature? PbI2(s) Pb2+(aq) + 2I(Aq) Ksp = [Pb2+] [ I ] 2 [1.2 X10 -3] [1.2 X10 -3]2 IV. Calculating Solubility from Ksp Calculate the solubility (in grams per liter) of calcium fluoride in water Ksp = 3.4 X 10-11 Calcium fluoride is commonly used as a window material for both infrared and ultraviolet wavelengths, since it is transparent in these regions (about 0.15 µm to 9 µm) and exhibits extremely weak birefringence. Calculating Solubility from Ksp CaF2(s) Ca+2(aq) + 2FSt 0 0 Ch +x +2X Eq +x +2X Ksp 3.9 X 10-11 = [Ca+2] [2F-]2 3.9 X 10-11 = [x] [2x]2 3.9 X 10-11 = 4x3 x = 2.1 x 10-4(78.1g/mol) = 1.6X10-2g/liter V. Factors Effecting Solubility There are five main factors that control solubility of a solute. (1) Temperature (2) Nature of solute or solvent (3) Pressure (4) common ion effect (5) pH Temperature Generally solubility increases with the rise in temperature and decreases with the fall of temperature but not in all cases. In endothermic process solubility increases with the increase in temperature and vice versa. In exothermic process solubility decrease with the increase in temperature. Gases are more soluble in cold solvent than in hot solvent. NATURE OF SOLUTE AND SOLVENT A polar solute dissolved in polar solvent. Solubility of a non-polar solute in a solvent is large. A polar solute has low solubility or insoluble in a non-polar solvent. In general like dissolves like EFFECT OF PRESSURE The effect of pressure is observed only in the case of gases. An increase in pressure increases of solubility of a gas in a liquid. For example carbon dioxide is filled in cold drink bottles (such as coca cola, Pepsi 7up etc.) under pressure. Common Ion Effect The presence of other solutes can also influence solubility – although they do not effect Ksp CaF2(s) Ca+2(aq) + 2Faddition of calcium or fluoride ion will shift the equilibrium left favoring solid formation and decreasing solubility of the compound Cal the sol of CaF2 at 25oc in a) 0.01 M Ca(NO3)2 b) 0.01M NaF Ksp 3.4 X 10-11 = [Ca+2] [2F-]2 CaF2(s) Ca+2(aq) + 2FSt 0 0 Ch +x +2X Eq 0.10+x +2X 3.9 X 10-11 = [0.010+x] [2X]2 assume that X is very small compared to 0.01 so 3.9 X 10-11 = (0.010) 4x2 3.9 X 10-11/ (0.010) 4 = x2 x = 3.1x10-5 mol/L In pure water the molar sol of CaF2 was 2.0 x 10-4 mol/L so we see the sol has decreased with the addition of a common ion. Learning check calc part b). Answer 3.9 x 10-7 mol/L b) 0.01M NaF CaF2(s) Ca+2(aq) + 2FSt 0 0 Ch +x +2X Eq +x 0.1+2X Assume x is small ignore 2X 3.9 X 10-11 = x(0.010) 2 X = 3.9 X 10-11 / (0.010) 2 X = 3.9 x 10-7 seems correct because F ion conc. is squared and has more of an effect