ADDITIONAL AQUEOUS EQUILIBRIA CHAPTER 17
advertisement

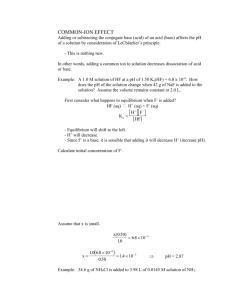
Additional Aqueous Equilibria CHAPTER 16 I. Buffers A. Definitions Buffer- solutions that resist changes in pH when acid or base are added to it. - they are composed of a weak acid and its conjugate base or a weak base and its conjugate acid. acetic acid / sodium acetate carbonic acid / carbonate B. Explanation of Buffers 1. Shifting of Equilibrium For some weak acid and its conjugate base: HA ⇌ H+ + Aa. What happens if you add excess acid (H+)? b. What happens if you add excess base (OH-)? ** Best buffering capacity when approximately equal amounts of both acid and conjugate base. Why strong acids and bases not good buffers? 2. Titration Curve For Buffer Acetic acid / acetate (CH3COOH / CH3COO-) 3. Mathematical Relationship a. Henderson-Hasselbalch Equation For: HA H+ + A[ H + ][ A- ] Ka = [HA] [ A- ] pH = pK a + log [HA] When [HA] = [A-], it is true that pH = pKa Best buffering region = ±1 unit of pKa C. Buffer Problem Solving Similar to other equilibrium problems, except we will use the Henderson Hasselbalch Equation base pH pKa log acid Always utilizes assumption that we can neglect small amount of dissociation of weak acid or base!!!!!!!! Different from Tro text, all our buffer problem solving will be done using the Henderson-Hasselbalch!!!! 1. Determining the pH of a Buffer Solution What is the pH of a buffered solution that is 0.015 M acetic acid and 0.045 M sodium acetate? For acetic acid, Ka = 1.8 x 10-5 ? 2. Preparation of a Buffer Solution a. Which acid-base pair should be used to prepare a buffer pH 5.0? Acetic acid / acetate Ka = 1.8 x 10-5 Ammonium / ammonia Ka = 5.6 x 10-10 b. A buffer solution pH 5.0 is to be prepared using acetic acid (Ka = 1.8 x 10-5) and sodium acetate . How many grams of sodium acetate (NaC2H3O2) should you add to 250.0 mL of a 0.0500 M acetic acid (HC2H3O2) solution? 3. Addition of Acid or Base To A Buffer A buffer is prepared by adding 0.30 mol of lactic acid and 0.40 mol of sodium lactate to sufficient water to make 2.00 L of buffer solution. The Ka of lactic acid is 1.4 x 10-4. (Assume Ka small enough to neglect dissociation of weak acid). a) Calculate the pH of the buffer. b) Calculate the pH of the buffer after the addition of 0.050 moles of NaOH. When Adding Strong Acid or Base To Buffer: 1. Addition of strong acid to buffer → converts a stoichiometric amount of the base to the conjugate acid. 2. Addition of strong base to buffer → converts a stoichiometric amount of the acid to the conjugate base. (Adding acid creates more acid, adding base creates more base) CONCEPT QUESTION A 1.00 liter buffer solution is 0.10 M in HF and 0.050 M in NaF. Which action will destroy the buffer? Why? a. addition of 0.050 mol HCl b. addition of 0.050 mol NaOH c. addition of 0.050 mol NaF d. addition of 0.05 mol HF D. Buffer Capacity 1. Definition- amount (moles) of acid or base required to change the pH of a given volume of buffer by ±1 pH unit. A measure of the ability of a buffer to resist changes in pH. Addition of more acid or base will cause the buffer to fail, meaning inability to resist changes in pH. II. Acid-Base Titrations A. Titration Process 1. Discuss Process (acid + base salt + water) 2. Equivalence Point: point in titration when stoichiometric amounts of titrant have been added to neutralize acid or base titrated. 3. Endpoint: point where titration is actually ended because of indicator color change. (Endpoint is used to visually determine equivalence pt.) B. Explanation of Acid-Base Indicators 1. Weak organic acids which undergo color change when there is a shift from acid base at complete neutralization. HIn (aq) + H2O (l) ⇌ H3O+ (aq) + In-(aq) acid one color base another color 2. Different indicators change colors at different pH’s Bromocresol green: pH 3.8 to 5.4 Phenolphthalein: pH 8.2 to 10 C. Titration of Strong Acid with Strong Base Example: HCl + NaOH H2O + NaCl Strong Acid-Strong Base Titration Problem A 50.00 mL sample of 0.100 M HCl is titrated with a 0.100 M NaOH solution. a. Determine the pH of the HCl solution before the addition of any NaOH solution. a. Determine the pH after the addition of 25.00 mL of the NaOH solution. b. Determine the pH after the addition of 50.00 mL of the NaOH solution. D. Titration of Weak Acid with Strong Base Example: HC2H3O2 + NaOH C2H3O2- Na+ + H2O Weak Acid-Strong Base Titration Problem A 50.00 mL sample of 0.100 M acetic acid (HC2H3O2, Ka = 1.8 x 10-5) is titrated with a 0.100 M NaOH solution. a. Determine the pH of the solution before the addition of the NaOH solution. b. Determine the pH after the addition of 50.00 mL of the NaOH solution. E. Titration of Weak Base with Strong Acid Example: NH3 + HCl NH4+ Cl1. Is the equivalence point less than or greater than pH 7? Explain you answer. 2. Draw a titration curve for the titration. (titrate NH3 using HCl as the titrant) F. Titration of Polyprotic Acid with Strong Base Example: H3PO4 III. Solubility Equilibria A. Equilibrium Expressions for Sparingly Soluble Salts 1. Ksp : Solubility Product Constant (Write like a normal Kc and recall it does not include solids in the equilibrium expression). Meaning: idea of extent of solubility of salt Larger Ksp Greater solubility of salt Write the chemical equations and Ksp expressions for the slightly soluble salts: AgCl CaCO3 Ag3PO4 Mg(OH)2 2. Solubility: amount of substance dissolved based on grams / volume. 3. Molar Solubility: maximum amount of substance dissolved based on moles / liter. Molar solubility is not the same as Ksp !!!!! The molar solubility of BaF2 is 6.27 x 10-3 M. The Ksp of BaF2 is 9.8 x 10-7. What is the concentration of F- ions in a BaF2 saturated solution? B. Problem Solving 1. Calculating Ksp from Solubility Data One liter of water is able to dissolve 2.15 x 10-3 mol of PbF2 at 250C. What is the molar solubility of PbF2? What is the Ksp for PbF2? 2. Calculating Molar Solubility From Ksp Determine the molar solubility of barium phosphate, Ba3(PO4)2, given a Ksp value of 3.4 x 10-23. What are the equilibrium concentrations of each ion in solution? 3. Common Ion Effect (Factor Affecting Solubility) Ag2CO3 has a Ksp of 8.1 x 10-12 at 250C. The molar solubility of Ag2CO3 in water is 1.3 x 10-4 M. What is the molar solubility of Ag2CO3 in a 0.10 M NaCO3 solution. (NaCO3 is very soluble). a. Qualitative Explanation – Le Chatelier’s Principle Ag2CO3 (s) 2 Ag+ (aq) + CO32- (aq) What is the effect of adding excess CO32- ? What is the effect on the molar solubility of Ag2CO3 ? b. Terminology Used: Common Ion: the ion which is common to both salts being considered. Common Ion Effect: the solubility of a compound is always lowered due to the addition of the common ion. Now solving problem: 4. Predicting If Precipitation Occurs Comparison of known Ksp to the calculated ion product (Q) for a given mixture can serve to indicate whether or not a precipitate will be formed. Q Ion Product > Ksp ppt. forms Ion Product = Ksp ppt. forms Ion Product < Ksp no ppt. forms Will a precipitate form at equilibrium when 50.00 mL of 0.00100 M BaCl2 is added to 50.00 mL of 0.000100 M Na2SO4 ? The solubility product constant for barium sulfate is 1.1 x 10-10.