Chapter 15 Applications of Aqueous Equilibria
advertisement

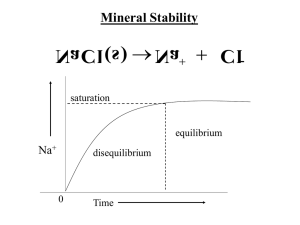
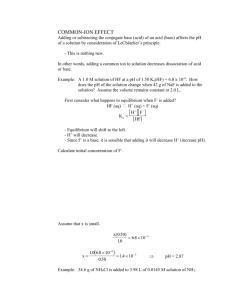
Chapter 15 Applications of Aqueous Equilibria • Some of what we do in this chapter will be the same as what we did in Chapter 14. • Let’s start with common ions. What do the weak acid HF and the salt NaF have in common? The common ion effect • When the salt with the anion of a weak acid is added to that acid: – it reverses the dissociation of the acid – Lowers the % dissociation of the acid • This same principle applies to salts with the cation of a weak base as well. • Calculations are the same as last chapter 15.2 Buffered solutions • When an acid-base solution contains a common ion, it’s called a buffered solution. • A buffered solution is one that resists a change in pH with the addition of hydroxide ions or protons. • Often buffered solutions contain a weak acid and it’s salt (HF and NaF) OR a weak base and it’s salt (NH3 and NH4Cl). • We can make a buffer of any pH by varying the concentrations of these solutions. Finding the pH of a buffered solution • Calculate the pH of a 1L buffered solution of 0.20M acetic acid solution and 0.30M sodium acetate. Ka=1.8x10-5 1 .8 x1 0 5 1 .8 x1 0 5 H C 2H 3 O 2 x 0 .3 x 0 .2 x H C 2H 3 O 2 0 .3 x 0 .2 x 1 .2 x1 0 p H lo g (1 .2 x1 0 5 ) 4 .9 2 5 pH changes in a buffered solution • We will use the solution from the last problem, but add 0.01M NaOH. Compare the pH change that occurs with the addition of the NaOH solid. • There are 2 major step to proceed with these types of problems!! – 1. The stoichiometry – 2. The equilibrium The stoichiometry 1L of 0.20M acetic acid solution and 0.30M sodium acetate (Ka=1.8x10-5 ) [H+]=1.2x10-5 Buffered with .01M NaOH • Use moles not molarity Before reaction After reaction HC2H3O2 OH- ↔ H 2O 0.20 mol 0.01 0.30 mol 0.20 – 0.01 =0.19mol 0 0.30+0.01 0.31mol + C2H3O2- • We will assume that all of the NaOH will be consumed Now for the equilibrium - ICE Before reaction After reaction HC2H3O2 OH- ↔ H 2O 0.20 mol 0.01 0.30 mol 0.20 – 0.01 =0.19mol 0 0.30+0.01 0.31mol + C2H3O2- • Since there is 1L of solution Concentration Initial Change Equilibrium HC2H3O2 H+ ↔ + C2H3O2- 0.19M 0 0.31M -x +x +x 0.19-x X 0.31+x 1 .8 x1 0 5 1 .8 x1 0 5 H C 2H 3 O 2 x 0 .3 1 x 0 .1 9 x H C 2H 3 O 2 0 .3 1x 0 .1 9 pH log(1.1x10 x 1 .1x1 0 5 5 ) 4.96 pH change = 4.96 – 4.92 (from previous problem) Change in pH = .04 with the addition of a buffer. Think about what would have happened…. • If base was added to just water K w H O H H 0 .0 1 H 1x1 0 1 2 1x1 0 14 • That means the pH of 0.01M NaOH in water is 12 • Minus the pH of water (7) means a change in pH of 5. • Compare adding NaOH to a buffered solution vs. an unbuffered solution….5 vs 0.04… Remember • Buffered solutions are simply solutions of weak acids and weak bases containing a common ion • When a strong acid is added to a buffered soltuion…You know how to do these problems. Just remember the two steps… – Stoichiometry first – ICE (equilibrium) second How does the buffer work? • Solve the equilibrium expression for [H+] H A Ka H A H K a H A A • This means the [H+] depends on the ratio of [HA]/[A-]…if you take the –log of both sides…(I won’t bore you with the math)… you get… A p H p K a lo g H A It has a name! A p H p K a lo g H A • It’s called the Henderson-Hasselback equation! • It also works for an acid and its salt, like HNO2 and NaNO2 • Or a base and its salt, like NH3 and NH4Cl, but you must remember to convert Ka to Kb in the equation An assumption A p H p K a lo g H A • One assumption of this formula is that the initial concentrations and the equilibrium concentrations are equivalent. (<5% validity) • It is a pretty safe assumption since the initial concentrations of HA and A- are relatively large in a buffered system. Let’s try • What is the pH of a solution containing 0.30M HCOOH and 0.52M KCOOH (formic acid and potassium formate) Ka=1.8x10-4 0 .5 2 4 p H lo g (1 .8 x1 0 ) lo g 0 .3 0 pH 3.77 0.24 4.01 Given base, salt, and Kb • Calculate the pH of 0.25M NH3 and 0.40M NH4Cl. (Kb = 1.8 x 10-5) • Don’t forget to find Ka FIRST!! And the ratio is base over acid.. Ka 1x10 14 1.8 x10 5 p H lo g (5 .6 x1 0 5.6 x10 10 10 0 .2 5 ) lo g 0 .4 0 p H 9 .0 5 15.3 Buffer Capacity • The pH of a buffered solution is determined by the ratio of [A-]/[HA]. • If the ratio doesn’t change much…then the pH won’t change much either • The more concentrated these two are, the more H+ and OH- the solution will be able to absorb. • Larger concentrations = bigger buffer capacity Try this problem. • Calculate the change in pH that occurs when 0.040 mol of HCl(g) is added to 1.0 L of each of the following: Ka= 1.8x10-5 5.00 M HAc and 5.00 M NaAc 0.050 M HAc and 0.050 M NaAc • Calculate initial pH for each solution (henderson-hasselbalch) p H lo g (1 .8 x1 0 5 5 ) lo g 4 .7 4 5 Now find the change after adding 0.04mol of HCl to 1.0L of solution HC2H3O2 Before reaction After reaction H+ ↔ + C2H3O2- 5.0 mol 0.04 5.0 mol 5.0 + 0.04 =5.04 0 5.0 - 0.04 =4.96 p H lo g (1 .8 x1 0 5 4 .9 6 ) lo g 4 .7 4 5 .0 4 • There’s virtually NO change. Now look at solution B. We already know the initial pH. Solution B is 1L of 0.050 M HAc and 0.050 M NaAc HC2H3O2 Before reaction After reaction H+ ↔ + C2H3O2- 0.050 mol 0.04mol 0.050 mol 0.050 + 0.04 =0.09 0 0.050 - 0.04 =0.01 p H lo g (1 .8 x1 0 5 0 .0 1 ) lo g 3 .7 9 0 .0 9 • Compared to 4.74 before the acid was added. Solution A contains much larger quantities of buffering components and has a larger buffering capacity than solution B Larger concentrations = bigger buffer capacity • Here’s why A p H p K a lo g H A Buffer Capacity • The best buffers have a ratio [A-]/[HA] = 1 • This is the most resistant to change and true only when [A-] = [HA] • Makes pH = pKa (since the log of 1=0) 10.5 Titrations and pH Curves • Titration is commonly adding a solution of known concentration until the substance being tested is consumed. • This is often noted by a color change and is called the equivalence point. • This is often observed in a graph of pH vs mL of titrant and is called a titration curve. Strong acid with a strong base titration • Net ionic equation H+ + OH- H2O • To know the amount of H+ at any point in the titration, we must determine the amount of H+ remaining and divide by the total volume of the solution. • Let’s first consider a new unit for molarity that is smaller as most titrations usually deal with mL Millimole (mmol) = 1/1000 mol Molarity = mmol/mL = mol/L The pH Curve for the Titration of 50.0 mL of 0.200 M HNO3 with 0.100 M NaOH Where all the H+ ions originally present, have reacted with all the OH- ions added Things to note • You need to do the stoichiometry for each step • mL x Molarity = mmol • There is no equilibrium. Both the acid and base dissociate completely • Use [H+] or [OH-] to figure pH or pOH • The equivalence point is when you have an equal number of moles of H+ and OH• In other words enough H+ is present to react exactly with the OH• Before the equivalence point H+ is in excess • After the equivalence point OH- is in excess The pH Curve for the Titration of 100.0 mL of 0.50 M NaOH with 1.0 M HCI Strong base titrated with strong acid. • Very similar to strong acid with a strong base except before the equivalence point the OH- is in excess and H+ is in excess after the equivalence point. The pH Curve for the Titration of 50.0 mL of 0.100 M HC2H3O2 with 0.100 M NaOH weak acid – strong base At equivalence point (pH > 7): A- is basic Titrating a weak acid with a strong base • Again, there is no equilibrium • Do the stoichiometry – the reaction of OHwith the weak acid is assumed to run to completion & the concentrations of the acid remaining & conjugate base are determined. • Determine the major species • Since HA is stronger than H2O it is the dominant equilibrium • Then do the equilibrium (HendersonHasselbach) The pH Curve for the Titrations of 100.0mL of 0.050 M NH3 with 0.10 M HCl weak base – strong acid At equivalence point (pH < 7): NH4+ is acidic In a titration curve • Equivalence point is defined by the stoichiometry NOT by the pH. • Remember it is where the mol of H+ are equal to the moles of OH- Summary • Strong acid and base just stoichiometry. • Weak acid with 0 ml of base - Ka • Weak acid before equivalence point –Stoichiometry first –Then Henderson-Hasselbach • Weak acid at equivalence point- Kb -Calculate concentration • Weak acid after equivalence - leftover strong base. -Calculate concentration Summary • Weak base with 0 ml of acid - Kb • Weak base before equivalence point. –Stoichiometry first –Then Henderson-Hasselbach • Weak base at equivalence point Ka. -Calculate concentration • Weak base after equivalence – left over strong acid. -Calculate concentration 15.5 Acid-Base Indicators • Two common methods for monitoring the pH – 1. pH meter – 2. acid-base indicator (this is not a good choice for finding the equivalence point.) Careful selection of indicator can help get results close. Endpoint IS change in color Equivalence point IS moles acid = moles base HIn • Most indicators are themselves a weak acid. They are one color with the proton attached and another without the proton. • Phenolphthalein, the most common indicator, is colorless as an acid and pink as a base (In-) Did the color change? • Typically, 1/10 of the initial form must be converted for the human eye to see a new color. When titrating an acidic solution… Ka H In H In In 1 H In 1 0 pH pKa 1 If a basic solution is titrated • Indicator will initially exist as In- and more HIn will form when acid is added Ka H In H In In 10 H In 1 pH pKa 1 Which indicator to use? • It is best to choose an indicator whose endpoint is closest to our equivalence point. • It is easier to choose an indictor if there is a large change in pH near the equivalence point (vertical area of pH curve) • The weaker the acid, the smaller the vertical area around the equivalence point, giving less flexibility in indicator choice 15.6 Solubility Equilibria and Ksp • Will it dissolve, and if not, how much? dissolved solid • If everything dissolves, it is an equilibrium position • Partial dissolving -- The solid will precipitate as fast as it dissolves (equilibrium) • Surface area changes the rate at which something dissolves, but NOT the amount (no change in equilibrium position) Generic equation M aN m b s aM ( aq ) bNm • M+ is the cation (usually metal) • Nm- is the anion (a nonmetal) • The solubility product for each compound is found below a K sp M N m b ( aq ) Solubility vs. solubility product • Solubility is NOT the same as solubility product. • Solubility product is an equilibrium constant. • It doesn’t change except with temperature. • Solubility is an equilibrium position for how much can dissolve. • A common ion can change this. Let’s try a couple… first an easy one! • What is the Ksp value of copper (I) bromide with a measured solubility of 2.0 x 10-4 mol/L. C u B r( s ) C u CuBr Cu+ ↔ + Br- I 2x10-4 0 0 C solid +2x10-4 +2x10-4 E solid 2x10-4 2x10-4 (aq ) Br K sp (2 x1 0 (aq ) 4 1 ) (2 x1 0 K sp 4 x1 0 8 4 1 ) Now a bit more difficult • Calculate the Ksp for bismuth sulfide, which has a solubility of 1.0x10-15M at 25oC B i 2 S 3 ( s ) 2B i Bi2S3 I 1x10-15 C solid E solid ↔ 2Bi+3 0 + S-2 0 +2(1x10-15) +3(1x10-15) 2x10-15 3x10-15 3 (aq ) 3S K sp (2 x1 0 15 2 (aq ) 2 ) (3 x1 0 K sp 1 .1x1 0 73 15 ) 3 Solubility from Ksp • Find the solubility of Silver Chloride. Ksp=1.6x10-10. A g C l( s ) A g ( aq ) K sp A g C l 1 .6 x1 0 10 1 .6 x1 0 x x 10 1 .3 x1 0 5 x x 2 Cl ( aq ) One more…a bit tougher • Find the solubility of copper (II) iodate, Cu(IO3)2. The Ksp for Cu(IO3)2 = 1.4x10-7 C u(IO 3 ) 2 ( s ) C u 2 K sp C u 2(IO 3 ) 1 .4 x1 0 7 1 .4 x1 0 3 x 2 x 7 3 .5 x1 0 4x 8 3 .3 x1 0 3 3 x 2IO 3 (aq ) 2 2 3 x 3 The solubility of copper (II) iodate is 3.3x10-3 (aq ) Comparing Ksp values • Ksp values give info about solubility (relative solubility). 1. When salts have the SAME number of ions: the larger the Ksp the more soluble 2. When salts have different number of ions: you CANNOT compare directly. Common ion effect and solubility • The presence of a common ion DECREASES the solubility of the salt. Let’s compare: What is the solubility of AgBr in both pure water & in 0.0010M NaBr. (pure H2O first) A g B r( s ) A g K sp 7 .7 x1 0 7 .7 x1 0 13 7 .7 x1 0 (aq ) 13 x x 13 8 .8 x1 0 7 x x 2 Br (aq ) Now with a common ion What is the solubility of AgBr in 0.0010M NaBr N a B r( s ) N a A g B r( s ) A g (aq ) Br (aq ) B r (aq ) Br 0 .0 0 1M (aq ) K sp 7 .7 x1 0 13 13 x x .0 0 1 AgBr ↔ Ag+ I 7.7x10-13 0 0.0010 a ssu m e x is n e g lig ib le C solid +x +x in x + 0 . 0 0 1 0 E solid x .0010x + Br- 7 .7 x1 0 7 .7 x1 0 13 0 .0 0 1 0 x 7 .7 x 1 0 10 x Compare the two results In Water K sp 7 .7 x1 0 7 .7 x1 0 13 7 .7 x1 0 13 K sp 7 .7 x1 0 x x 13 8 .8 x1 0 Common ion solution 7 x x 2 7 .7 x1 0 13 13 x x .0 0 1 a ssu m e x is n e g lig ib le in x + 0 . 0 0 1 0 7 .7 x1 0 13 0 .0 0 1 0 x 7 .7 x 1 0 10 x Notice that the common ion reduces the solubility as stated earlier (the reverse reaction occurs faster: equilibrium lies to the left) pH and solubility • Presence of a common ion decreases solubility Insoluble bases dissolve in acidic solutions • Insoluble acids dissolve in basic solutions add remove 2 M g(O H ) 2 ( s ) M g 2O H ( aq ) ( aq ) K s p 1 .2 x1 0 K s p M g 1 .2 x1 0 1 .2 x1 0 2 11 O H 11 s( 2 s ) 11 4s s 1 .4 x1 0 2 2 3 4 O H 2 s 2 .8 x1 0 4 p O H 3 .5 5 & p H 1 0 .4 5 At pH less than 10.45 Lower [OH-] Increase solubility of Mg(OH)2 At pH greater than 10.45 Raise [OH-] Decrease solubility of Mg(OH)2 15.7 Precipitation – mixing two solutions to get a solid • Let’s look at the reverse process of dissolving salts to form a solution. • Ion product is Q M a N m b • Initial concentrations are used Q < Ksp Unsaturated solution No precipitate Q = Ksp Saturated solution Q > Ksp Supersaturated solution Precipitate will form Try this • A solution of 750.0 mL of 4.00 x 10-3M Ce(NO3)3 is added to 300.0 mL of 2.00 x 10-2M KIO3. Will Ce(IO3)3 (Ksp=1.9x10-10M) precipitate and if so, what is the concentration of the ions? • Step 1: find the concentration of each of the ions expected to precipitate • Step 2: Find Q • Step 3: compare to Ksp




![K sp = [Pb 2+ (aq)][Cl](http://s2.studylib.net/store/data/005788724_1-fd79e2539544b4374a3f7aa03b8a844b-300x300.png)