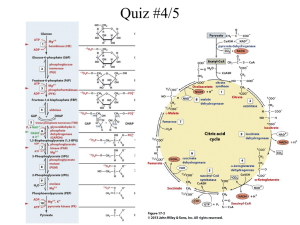
Chapter 8: Energy and Metabolism
advertisement

BIOL 1020 – CHAPTER 8 LECTURE NOTES Chapter 8: An introduction to metabolism Why do organisms need energy? How do organisms manage their energy needs? I. Energy and thermodynamics A. Living organisms require energy to do work, any change in state or motion of matter 1. energy can be expressed in units of work (kJ) or heat energy (kcal); 1 kcal = 4.184 kJ 2. energy can change forms (energy conversion) 3. organisms carry out transformation in energy forms between potential energy (capacity to do work) and kinetic energy (energy of motion, actively performing work) 4. organisms commonly use chemical bonds for storage and transfer of (potential) energy 5. work is required for the processes of life B. Two laws of thermodynamics describe the constraints on energy usage 1. First law: the total amount of energy (+ matter) in a closed system remains constant (principle of conservation of energy) The universe is a closed system Living things are open systems 2. Second law: in every energy conversion, some energy is converted to heat energy that is lost to the surroundings, and thus cannot be used for work Every energy conversion increased the entropy of the universe. Energy converted to heat in the surroundings increases entropy (spreading of energy) no energy conversion is 100% efficient organisms must get a constant influx of energy because of energy is lost in conversions II. Metabolic reactions include anabolism and catabolism, and involve energy transfers A. Recall that metabolism is the sum of chemical activities in a organism B. Metabolism can be divided into anabolism (anabolic reactions) and catabolism (catabolic reactions) 1. anabolic reactions are processes that build complex molecules from simpler ones 2. catabolic reactions are processes the break down complex molecules into simpler ones C. Chemical reactions involve changes in chemical bonds and substance concentrations, along with changes in free energy 1. free energy = energy available to do work in a chemical reaction (such as: create a chemical bond) free energy changes depend on bond energies and concentrations of reactants and products bond energy = energy required to break a bond; value depends on the bond left undisturbed, reactions will reach dynamic equilibrium when the relative concentrations of reactants and products is correct forward and reverse reaction rates are equal; concentrations remain constant cells manipulate relative concentrations in many ways, so that equilibrium is rare for key reactions 2. exergonic reactions – the products have less free energy than reactants the difference in energy is released and is available to do work exergonic reactions are thermodynamically favored; thus, they are spontaneous, but not necessarily fast (more on activation energy later) catabolic reactions are usually exergonic ATP + H2O ADP + Pi is highly exergonic in cellular conditions 3. endergonic reactions – the products have more free energy than the reactants the difference in free energy must be supplied (stored in chemical bonds) endergonic reactions are not thermodynamically favored, so they are not spontaneous an endergonic reaction is coupled with an exergonic reaction to provide the needed energy to drive an endergonic reaction together, the coupled reactions must have a net exergonic nature reaction coupling requires that the reactions share a common intermediate(s) EXAMPLE: A B (exergonic) C D (endergonic) Coupled: A + C B + D (overall exergonic) Actually: A + C I B + D typically, the exergonic reaction in the couple is ATP + H2O ADP + Pi anabolic reactions are usually endergonic 1 of 4 BIOL 1020 – CHAPTER 8 LECTURE NOTES One way that organisms manage their energy needs is to use ATP as a ready energy source for many reactions. III. ATP is the main energy currency in cells A. Recall ATP (adenosine triphosphate) is a nucleotide with adenine base, ribose sugar, and a chain of 3 phosphate groups B. The last two phosphate groups are joined to the phosphate group chain by unstable bonds; breaking these bonds is relatively easy, and releases energy; thus: 1. hydrolysis of ATP to ADP and inorganic phosphate (P i) releases energy ATP + H2O ADP + Pi 2. the amount of energy released depends in part on concentrations of reactants and products, but is generally ~30 kJ/mol C. Intermediates are involved when ATP hydrolysis is coupled to a reaction to provide energy; often these involve phosphorylated compounds, with the inorganic phosphate removed from ATP transferred onto another compound rather than being immediately released EXAMPLE: glucose + fructose sucrose + H2O (endergonic; requires ~27 kJ/mol) ATP + H2O ADP + Pi (provides ~30 kJ/mol) coupled: glucose + fructose + ATP + H2O sucrose + H2O + ADP + Pi intermediates: glucose + fructose + ATP + H2O glucose-P + fructose + ADP sucrose + H2O + ADP + P simplified: glucose + fructose + ATP sucrose +ADP + Pi D. Thus, energy transfer in cellular reactions is often accomplished through transfer of a phosphate group from ATP E. Making ATP involves an endergonic condensation reaction 1. reverse of an exergonic reaction is always endergonic ADP + Pi ATP + H2O (endergonic, usually requires more than ~30 kJ/mol) 2. must be coupled with an exergonic reaction; typically from a catabolic pathway F. ATP is typically created in catabolic reactions and used in anabolic reactions, linking those aspects of metabolism G. Cells maintain high levels of ATP relative to ADP 1. maximizes energy available from hydrolysis of ATP 2. ratio typically greater than 10 ATP: 1 ADP H. Overall concentration of ATP still very low 1. supply typically only enough for a few seconds at best 2. instability prevents stockpiling 3. must be constantly produced 4. in a typical cell, the rate of use and production of ATP is about 10 million molecules per second 5. resting human has less than 1 g of ATP at any given time but uses about 45 kg per day Redox reactions are used to harvest energy from some chemicals; the acceptors of that energy typically cannot be used directly as energy currency. IV. Redox reactions are also used for energy transfer A. Electrons can also be used for energy transfer 1. Redox reactions: recall reduction, gain electrons; oxidation, lose electrons; both occur simultaneously in cells (generally no free electrons in cells) 2. Typically, the oxidized substance gives up energy with the electron, the reduced substance gains energy with the electron 3. Commonly occur as a chain of redox reactions or electron transfers (more on electron transport chains later) 4. As the electron is transferred to an acceptor molecule, it releases free energy that can be used for other chemical reactions 5. Typically, a proton is removed as well when an electron is removed from covalent molecules; thus, the equivalent of a hydrogen atom is transferred B. Catabolism typically involves removal of hydrogen atoms from nutrients (such as carbohydrates), and the transfer of the protons and electrons to intermediate electron acceptors 1. One common intermediate acceptor is nicotinamide adenine dinucleotide (NAD+) Use XH2 to represent a nutrient molecule: XH2 + NAD+ X + NADH + H+ Often, the reduced form is just called NADH Reduced state stores energy, which is partially released as free energy when NADH is oxidized The free energy usually winds up being used to make ATP 2. Other commonly used acceptors are NADP+, FAD, and cytochromes NADP+/NADPH – important in photosynthesis 2 of 4 BIOL 1020 – CHAPTER 8 LECTURE NOTES FAD/FADH2 – flavin adenine dinucleotide Cytochromes – small iron-containing proteins; iron serves as electron acceptor Enzymes are a large part of the answer to how organisms manage their energy needs. Manipulation of reactions is essential to and largely defining of life, and enzymes manipulate the speed of reactions. Understanding life requires understanding how enzymes work. V. Enzymes regulate chemical reactions in living organisms A. An enzyme is an organic molecule (typically a protein) that acts as a catalyst 1. catalyst – substance that increases the rate of a chemical reaction without being consumed in the reaction (the catalyst recycles back to its original state) 2. enzymes (catalysts) only alter reaction rate; thermodynamics still governs whether the reaction can occur – thus, enzymes only catalyze reactions that are occurring anyway B. Enzymes (catalysts) work by lowering the activation energy of a reaction 1. all reactions have a required energy of activation (the energy required to break existing bonds) that must be supplied in some way before the reaction can proceed; also, reactants must come together 2. catalysts greatly reduce the activation energy requirement, making it easier for a reaction to occur 3. often, this reduction in activation energy is due to in part to the enzyme holding reactants (substrates) close together, which also reduces the reliance on random collisions C. Enzymes lower activation energy by forming a complex with the substrate(s) 1. the ability to form an enzyme-substrate complex is highly dependent on the shape of the enzyme 2. the site where the substrate(s) binds to the enzyme is called the active site 3. when the enzyme-substrate complex forms, there are typically shape changes in the enzyme and substrate(s) – this is called induced fit 4. the enzyme-substrate complex is typically very unstable and short-lived; it breaks down into released product(s) and a free enzyme that is ready to be reused 5. overall: enzyme +substrate(s) ES complex enzyme + product(s) D. Enzyme names 1. many names give some indication of substrate 2. most enzyme names end in –ase (example: sucrase) 3. some end in –zyme (example: lysozyme) 4. some traditional names are less indicative of enzyme function (example: pepsin) E. Enzymes are generally highly specific 1. overall shape as well as spatial arrangements in the active site limit what enzyme-substrate complexes can readily form 2. the amount of specificity depends on the particular enzyme example of high specificity: sucrase splits sucrose, not other disaccharides example of low specificity: lipase splits variety of fatty acids from glycerol 3. enzymes are classified by the kind of reaction they catalyze F. Many enzymes require cofactors to function 1. apoenzyme + cofactor active enzyme (bound together) 2. alone, an apoenzyme or a cofactor has little if any catalytic activity 3. cofactors may or may not be changed by the reaction 4. cofactors can be organic or inorganic organic examples (coenzymes): ATP, NADH, NADPH, FADH2 typically changed by the catalyzed reaction inorganic examples metal ions like Ca2+, Mg2+, Fe3+, etc. typically not changed by the catalyzed reaction 5. most vitamins are coenzymes or part of coenzymes, or are used for making coenzymes G. Enzymes are most active under optimal conditions 1. each enzyme has an optimal temperature most effective as a catalyst at the optimal temperature rate of drop-off in effectiveness away from optimal temperature depends on the enzyme high temperatures tend to denature enzymes human enzymes have temperature optima near human body temperature (37°C) 3 of 4 BIOL 1020 – CHAPTER 8 LECTURE NOTES each enzyme has an optimal pH again, most effective at the optimum; drop-off varies extremes of pH tend to denature enzymes a particular organism shows more variety in enzyme pH optima than in temperature optima, but most of its enzymes will still be optimal at the pH normally found in the cytosol of its cells H. Metabolic pathways use organized “teams” of enzymes 1. the products of one reaction often serve as substrates for the next reaction 2. removing products (by having them participate the “next reaction”) improves reaction rate 3. multiple metabolic pathways exit in cells, overlapping in some areas and diverging in others I. Cells can regulate enzyme activity to control reactions 1. increase substrate amount increase reaction rate (up to saturation of available enzyme molecules) 2. increase enzyme amount increase reaction rate (as long as substrate amount > enzyme amount) 3. compartmentation of the enzyme, substrate, and products can help control reaction rate 4. inhibitors and activators of enzymes inhibitors reduce or eliminate catalytic activity activators allow or enhance catalytic activity sometime, this uses an allosteric site – a receptor site on an enzyme where an inhibitor or activator can bind a common example of allosteric control is feedback inhibition, where the last product in a metabolic pathway binds to an allosteric site of an enzyme in an early step of the pathway (often the first) and inhibits activity of the enzyme irreversible inhibition – enzyme is permanently inactivated or destroyed; includes many drugs and toxins reversible inhibition – if inhibitor is removed, the enzyme activity can be recovered competitive inhibition – inhibitor is similar in structure to a substrate; competes with substrate for binding to the active site noncompetitive inhibition – binds at allosteric site, alters enzyme shape to make active site unavailable 2. 4 of 4