Spectra - prettygoodphysics
advertisement

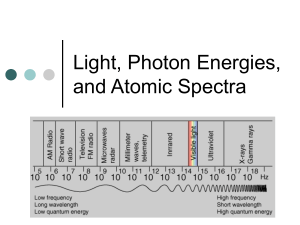
AP Physics Laboratory #22 Emission Spectra DISCUSSION: According to classical physics, as a wave light is characterized by its frequency (f) in Hertz (Hz) and its wavelength (λ), both of which are related to the speed of light (c): c f When solids are heated, they emit light. The light produced this way is called black-body radiation. As the temperature increases the color distribution shifts to higher frequency. In 1900 Max Planck proposed that the vibrating atoms in a heated solid could absorb or emit electromagnetic energy only in certain discrete amounts. Planck introduced an equation that relates the frequency of light to its energy. Planck’s equation is: E hf h is Planck's constant, 6.626 x 10–34 J • s. Planck hypothesized that energy can only be absorbed or emitted as positive whole number multiples of hf. That is, an energy change is allowed to be hf, 2hf, 3hf, and so on, but is not allowed to be a fraction of hf. Planck’s quantum theory introduced the idea that energy is quantized. Bohr derived an expression of energy levels specifically for the hydrogen atom: E n 2.179 x10 18 1 n2 Albert Einstein extended Planck’s theory to successfully explain the photoelectron effect. Einstein suggested that light could be described not only as having wave-like properties but also as having particle-like properties. He proposed that particles of light called photons are tiny packets of energy and that the energy of a photon of light with frequency f is given by Planck’s equation. E photon hf As a result of Einstein’s work we now view light as both a particle and a wave. The electric current applied to a cathode ray tube causes electrons to flow from the cathode to the anode. These electrons collide with the gas atoms in the tube causing electrons in the gas atom to jump from low energy levels to higher energy levels. When this happens, the atom is no longer in its ground state; it is in a higher energy excited state. Electrons have a natural tendency to return to the ground state by losing energy. This excess energy is lost through the emission of light. The energy of the emitted photon (light) corresponds to the energy lost by the electron as it moves from the higher energy level to a lower energy level. When an electron loses energy by undergoing a transition from a higher to a lower energy level in an atom the amount of energy lost will be ΔE: E E final Einitial where Efinal is the energy of the final state and Einitial is the energy of the initial state. When the energy is lost due to the emission of a photon of light by the atom, the frequency and wavelength of the light is related to ΔE as follows: E E photon hf hc THE EXPERIMENT: 1. Using your spectroscope look at the light being emitted by the hydrogen lamp. Use the colored pencils to record the emission spectrum that you see on the scale below. Write down the wavelengths of the emission lines that you observe below each line. 2. Carefully replace the hydrogen lamp with the mercury lamp. Using your spectroscope look at the light being emitted by the mercury lamp. Use the colored pencils to record the emission spectrum that you see on the scale below. Write down the wavelengths of the emission lines that you observe below each line. 3. Carefully replace the mercury lamp with the helium lamp. Using your spectroscope look at the light being emitted by the helium lamp. Use the colored pencils to record the emission spectrum that you see on the scale below. Write down the wavelengths of the emission lines that you observe below each line. ANALYSIS: In addition to any necessary measurements or calculations from the experiment itself, include the following in your lab report. 4. Using Bohr’s equation for the allowed electron energy levels of the hydrogen atom calculate the energy for the first six electron energy levels. The energies for n = 1 and n = 2 are already done for you. It is useful to write all of the energies to the same power of ten (use 10–19 as indicated). Value of n Energy in J 6 5 4 3 2 5.448 x 10–19 1 21.79 x 10–19 5. Complete the table given below by calculating the photon energy in joules and wavelength in nanometers for each electron transition of hydrogen indicated. The energies should all be reported to the same power of ten (use 10–19 as indicated). The ninital = 2 to nfinal = 1 transition is already done for you. Transition ninitial nfinal 62 61 54 53 52 51 43 42 41 32 31 21 Energy ( x 10-19 J) Wavelength (nm) 16.34 121.6 6. Referring to the table you already completed and your data for the emission spectrum of hydrogen identify which transitions correspond to the emission lines that you observed for hydrogen. Wavelength Observed (nm) Transition ninitial nfinal 7. Construct an energy level diagram to scale for the allowed energies of the electron in the hydrogen atom. A sketch of what the diagram will look like is given below: 8. On the energy level diagram: a. Draw and label the n = 1 to n = 6 energy levels. Give the value of n and the energy for each level. Do not forget to label your energy units! b. Indicate which energy level is the ground state. c. Indicate which transitions correspond to the emission lines you observed for hydrogen. d. Using colored pencils to indicate the color of the associated energy transition, draw the transitions as down arrows in your diagram. Label each transition with the wavelength that you observed. 9. Based on your results, what is nfinal for all of the visible emission lines of the hydrogen atom? 10. Consider a hydrogen atom with its electron in the n = 5 excited state. Which transition from this state will result in the emission of the lowest frequency of light? Explain your reasoning.