first midterm examination
advertisement
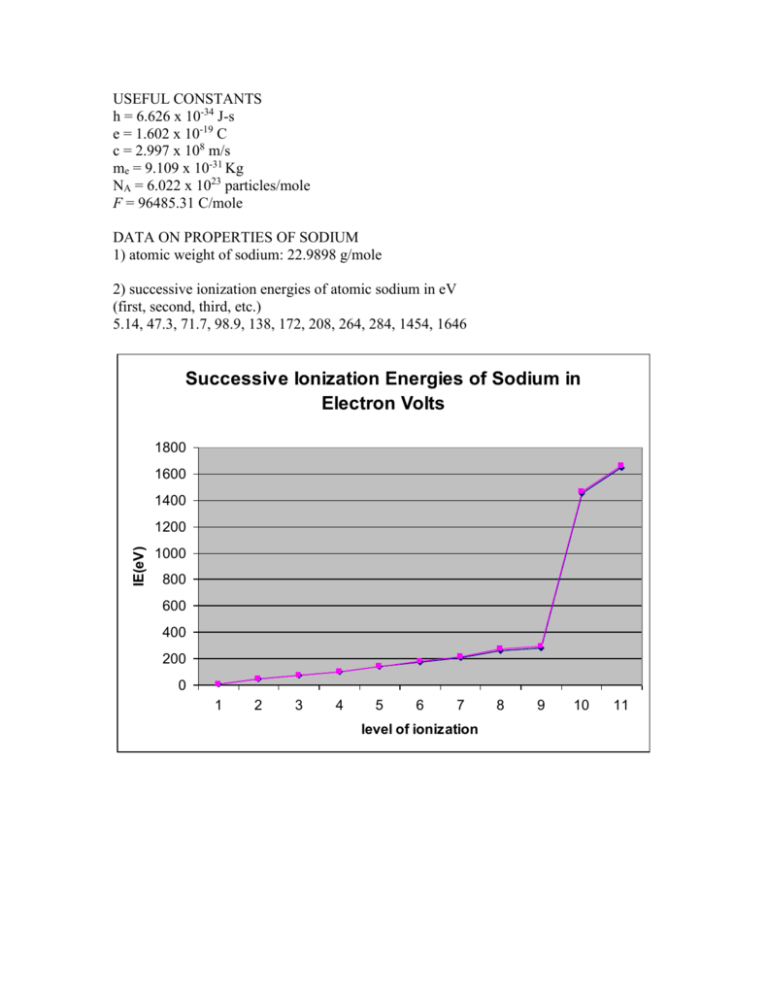
USEFUL CONSTANTS h = 6.626 x 10-34 J-s e = 1.602 x 10-19 C c = 2.997 x 108 m/s me = 9.109 x 10-31 Kg NA = 6.022 x 1023 particles/mole F = 96485.31 C/mole DATA ON PROPERTIES OF SODIUM 1) atomic weight of sodium: 22.9898 g/mole 2) successive ionization energies of atomic sodium in eV (first, second, third, etc.) 5.14, 47.3, 71.7, 98.9, 138, 172, 208, 264, 284, 1454, 1646 Successive Ionization Energies of Sodium in Electron Volts 1800 1600 1400 IE(eV) 1200 1000 800 600 400 200 0 1 2 3 4 5 6 7 level of ionization 8 9 10 11 NAME:___SOLUTIONS TO THE EXAM____ Chem 1b, 2005, Midterm 1 Useful constants and data can be found on the insert. You may use the insert for scratch work but enter all work to be graded in the space provided with each question. Show your work to receive credit in any problem involving a calculation or derivation. 1) (37 points) The element sodium (Z = 11) has a single stable isotope, 23Na. 24Na, an unstable isotope with a half life of 14.96 hours, is used as a radioactive tracer. a) Suggest a means of producing 24Na from readily available sources. Irradiate a sample of normal sodium which is entirely 23Na with neutrons, n + 23Na 24Na b) Provide a balanced nuclear equation for the decay of 24Na. 24Na has an excess of neutrons and the mostly likely decay channel is beta decay whereby a neutron converts into a proton. 24 Na 24 Mg+ + e- + Some students proposed the reverse of the nuclear reaction given in part (a). This is possible as all reactions can proceed in both directions. Beta decay is more likely. Another conceivable reaction is the emission of a stable alpha particle. This is normally observed with very large, unstable nuclei but not with smaller nuclides. c) A patient is injected with 5.0 milliCurie (1.44 x 1013 atoms) of 24Na. Calculate how many of atoms of 24Na remain after 2.0 days. The relationship for time dependence is N = N0exp(-kt) where k = ln(2)/. k = 0.693/(14.96 h) 0.04633 h-1 and t = (2 d)(24 h/d) = 48 h N = (1.44 x 1013 atoms)exp[-(0.04633 h-1)(48 h)] = 1.6 x 1012 atoms d) The decay of each atom of 24Na releases 5.5158 Mev of energy. Outline how this number can be calculated from data found in resources such as your textbook and the Handbook of Chemistry and Physics. The CRC handbook contains masses of the nuclides as well as masses of the elementary particles. From the above reaction, m = (m24Mg+ + me) - m24Na = (m24Mg - me + me) - m24Na = m24Mg - m24Na. 2) (65 points) The first ionization energy of atomic sodium is 5.14 eV (496 kJ/mole). a) Provide the n, l, ml, and s quantum numbers of the electron that is ionized. The outermost valence electron will be removed so n = 3, l = 0, ml = 0, s = 1/2. b) Suppose that the ionization is effected by irradiation with light. Calculate the maximum wavelength of light that will ionize the sodium. IE = 496,000 J/mole = (496,000)/(6.022 x 1023 atoms/mole) = 8.236 x 10-19 J/atom, IE = E(photon) = h so = IE/h = 1.243 x 1015 Hz. = c/ 2.41 x 10-7 m = 241 nm c) Calculate the effective nuclear charge of the electron that is ionized. IE = -E(electron in an orbital) = -E [Koopman's theorem] E = -RZe2/n2 so Ze = [IEn2/R]0.5 = [(5.14 eV)(32)/(13.6 eV)]0.5 = 1.84 d) Calculate the rms position of this electron. <r> = a0n2/Ze = (0.529 Å)(32)/(1.84) = 2.58 Å e) Will this electron ever be found at the nucleus? Briefly discuss. Yes. There is no angular momentum and hence no centrifugal force to oppose the natural electron-nuclear attraction that will draw the electron to the nucleus. f) Suppose that the electron is excited to a state in which n = 4, l = 2, and ml = 1. i) In units of h/2, calculate the value of L (orbital angular momentum) and Lz (the z-component of L) for the electron in the excited state. L = [l(l+1)]0.5 (h/2) = (6)0.5 h/2, Lz = ml (h/2) = 1 h/2 ii) Describe the symmetry of the distribution of this excited electron. The asymmetrical electron distribution is a consequence of non-zero angular momentum. The symmetry is given by the angular nodes which are planes in this case. With l = 2 (d orbital), there are two angular nodes. Since ml = 1, one of them contains the z axis. The other is perpendicular to the z axis; it is the xy plane. iii) When the excited 4d electron drops down to an energy level with n = 3, light with one of 3 wavelengths can result. Briefly explain why 3. In a multi-electron atom, the energy of an electron has its expected dependence on n but it also indirectly depends on l 3) (18 points) With the aid of a molecular-beam nozzle source, a stream of gaseous 23Na atoms is produced with a speed of 250 m/s. a) Calculate the de Broglie wavelength of the sodium atoms. = h/p. p = mv Use SI units and atomic quantities. m = (0.023 Kg/mole)/(6.022 x 1023 atoms/mole)= 3.82 x 10-26 Kg/atom = (6.62 x 10-34 J-s)/[(3.82 x 10-26 Kg/atom)(250 m/s)] = 6.94 x 10-11 m b) A platinum crystal whose atoms are arranged in planes with a separation of 3.921 Å is used as a grating in a diffraction experiment for measuring the de Broglie wavelength. The experiment will not demonstrate the quantummechanical wavelike properties of the sodium atoms. Briefly explain why. A successful, e.e. resolved interference fringes, requires that d be comparable with the de Broglie wavelength. The de Broglie wavelength is much smaller than d so a successful experiment is unlikely. 4) (15 points) The values of the eleven ionization energies (i.e. the first, second, etc.) of atomic sodium are given on the insert. Discuss how these data support the electron configuration of sodium and the shell model. One predicts from the shell model the configuration (1s)2(2s)2(2p)6(3s) which is well supported by the data. A high n (n=3) and a low effective nuclear charge leads to a small first ionization energy (IE). The next IE is much high as a core electron with n = 2 is removed. With the removal of the six 2p electrons, one observes a nearly linear increase in IE. This trend is due to the decrease with ionization in self screening by the electrons in the n = 2 shell. One observes a break in the slope with the removal of the 2s electron. The increase in IE is due to the larger effective nuclear charge for an s electron which has no orbital angular momentum and can spend part of its time at the nuclear. The IE is very large for the removal of the last two electrons for which n =1 and Z is at or close to Z = 11. 5) (15 points) Organize the atomic species (Na+, Na, Ne, and Na-) in order of increasing atomic radius and provide in a succinct essay the basis for your answer. The atomic number of neon (Ne) is 10. (You may wish to continue your essay on the back.) The order is Na+ < Ne < Na < Na-. Consider first the sodium species. Z = 11 for all of them. Na+ will be the smallest of the set as it contains only core electrons with n = 1 and 2. Na and Na- will be larger as they contain 1 and 2 valence electrons, respectively with n =3. Na- will be larger as the additional of an electron is not compensated by an increase in Z. Ne will be larger than Na+. The two species are isoelectronic but Na+ has the larger nuclear charge. 13 February 2005, WES, exam_1b_2005_1_soln.doc