LAB 3: SPECTROPHOTOMETRY- MEASURING ABSORPTION OF
advertisement

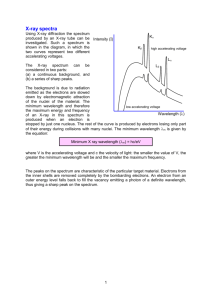
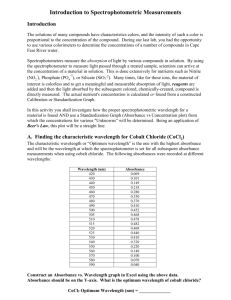
GISAT 112 Fall 2008 SPECTROPHOTOMETRY- MEASURING ABSORPTION OF LIGHT INTRODUCTION There is something magical about rainbows and colored light from glass prisms. The separation of white light into its colored components causes these visual phenomena. In this experiment you will investigate this partitioning of light in the visible region of the spectrum and its interaction with colored solutions. You will discover what colors correspond to what wavelengths and, for each of several colored solutions, you will collect the necessary data to construct a spectrum showing how light is absorbed at different wavelengths for that substance. Measurements of this kind are widely used in nearly all areas of the physical and biological sciences. An understanding of the electromagnetic spectrum and the interactions of radiation with matter is crucial to two of the case studies in Chemistry in Context: the stratospheric ozone shield (chapter 2) and global warming (chapter 3). BACKGROUND INFORMATION The electromagnetic spectrum is discussed in Section 2.4 of the text. The region that the human eye can detect with its wonderful rainbow of colors, the "visible" region, is only a small portion of the total electromagnetic spectrum. Each unique type of light in the electromagnetic spectrum is identified by its wavelength. For the visible region, the lengths of the light waves are in the range of billionths of a meter and therefore they are expressed as nanometers, nm. (1 nm = 1 x -9 10 meter). The instrument used to measure the interaction of light with matter is a spectrophotometer. Spectrophotometers are available for most regions of the electromagnetic spectrum. Those designed for the visible region of the spectrum were developed first and are still the most common. Figure 1 shows the optical diagram for a simple spectrophotometer of the type that you will use for this experiment. This device is designed to split visible light into its component colors and then allow the light to pass through a given sample of material. An electronic detector measures the amount of light that has been transmitted or absorbed by the sample at each wavelength. All spectrophotometers have essentially the same major components but with varying degrees of sophistication. These essential components are a light source, a device to isolate or resolve particular wavelengths of light, a sample holder, and a detector. There will also be some kind of meter or other device to display the measured transmittance or absorbance of light. Page 1 of 10 GISAT 112 Fall 2008 Figure 1. Optical diagram of a Spectronic 20 Spectrophotometer The major components shown in Figure 1 are: (a) the light source (lamp), (b) some lenses and a grating that are used to select a particular wavelength (actually a narrow band of wavelengths), (c) the sample to be measured (usually in a test tube-like container) and (d) a phototube that converts light intensity into an electrical signal that can be connected to a meter (not shown). A simple spectrophotometer of this type can only measure the transmittance of light at a single wavelength at a time. Obtaining data over a range of wavelengths requires multiple measurements, each made at a different wavelength. Because light intensity and detector sensitivity vary with wavelength, each measurement must be corrected using a blank. For this experiment, the blank is simply a tube containing pure water. The instrument has a scale labeled Percent Transmittance or %T. This scale is defined as the intensity of light that passes through a sample of known thickness (the "path length") divided by the light that passes through a blank of equal thickness, and multiplied by 100%: % Transmittance (%T) = transmittance by sample transmittance by blank x 100% In practice, this means you should use two matched test tubes or “cuvettes”: one containing the sample and one containing pure water. If the transmittance of the blank is first adjusted to read 100% at a particular wavelength, you can then read the percent transmittance of samples directly from the meter on the spectrophotometer. From your transmission data you will construct a graphical representation of the light transmittance of a colored compound over a range of wavelengths. Measuring the percent of the light transmitted at different wavelengths and plotting the wavelength on the x-axis of a graph and the % transmittance on the y-axis accomplish this. The result is a spectrum, a curved line that shows how a particular solution transmits light in the visible region of the electromagnetic spectrum. This spectrum is the result of qualitative analysis; it is characteristic of that compound in solution, and can be used to identify or detect the presence of that compound in other samples. The Spectronic 20 spectrophotometer also displays a different property, Absorbance. In a general way, absorbance is the opposite of transmittance. Thus, for example, if a sample Page 2 of 10 GISAT 112 Fall 2008 transmits all of the light at a certain wavelength, the absorbance of light by the sample at that wavelength must be zero. Conversely, if the transmittance is low, the absorbance is high. The mathematical relationship is slightly more complicated: absorbance is defined as the negative logarithm of the transmittance, or A = -log T. The particular utility of absorbance is that it is directly proportional to the concentration of the colored or absorbing material. Absorbance is therefore an important parameter for quantitative analysis, where the amount or concentration of a substance is determined. In this experiment, you will first observe the colors of different wavelengths of light throughout the visible region. By adjusting a wavelength dial you can select particular wavelengths and observe them as they are reflected off a white surface. You will record what colors you see, and what maximum and minimum wavelengths your eyes can detect. Then you will determine which wavelengths of visible light are transmitted or absorbed by various colored solutions. The colored liquids you will use for this part of the experiment are food-coloring dyes. Colored substances can be analyzed by this instrument as long as they meet two conditions: (a) they must be transparent so that light will pass through them, and (b) they need to have a fairly bright, distinct color. One category of colored substances of particular interest to chemists is acid-base "indicators", colored dyes that are used to identify the acidic or alkaline character of solutions. Your instructor may ask you to do a study of such an indicator, either in addition to or in place of the study of food coloring dyes. This will entail measuring the absorption spectrum of the indicator in both its acidic and alkaline forms. OVERVIEW OF THE EXPERIMENT 1. 2. 3. 4. Observe the color of light of selected wavelengths from 400 to 700 nm. Measure the transmittance spectra for red, blue, green, and unknown solutions. Plot a graph of transmittance vs. wavelength for each colored solution. Plot a graph of absorbance vs. wavelength, and compare with standard spectra that will be provided to you, to identify the unknown colored substance. 5. For extra credit: prepare a “standard curve” for the blue dye. MATERIALS NEEDED Chemicals · Solutions of food color dyes · Sample of a colored substance Equipment · A simple spectrophotometer · 5 plastic cuvettes (or test tubes) · 1 cuvette with white solid block · A holder for the cuvettes · 10-mL graduated cylinder Page 3 of 10 GISAT 112 Fall 2008 EXPERIMENTAL PROCEDURE [Note: The following instructions assume that you are using a Genesys 20 Spectrophotometer, which is one of the most widely available simple spectrophotometers. If you are using a different kind of spectrophotometer, your instructor will provide alternative instructions.] A. Looking at Light 1. Turn on the spectrophotometer using the on/off switch on the back of the unit. Press the A/T/C button until the screen displays %T. 2. Place the spectrophotometer cuvette with the white block in it into the spectrophotometer so that the slanted side is facing the back of the unit. 3. Press the wavelength controls (“nm▲” and “▼nm”) until the screen reads 500 nm. 4. Look down the tube at the white block. You should see a colored spot of light reflected off the block. If you still cannot see the colored spot, consult your instructor. 5. Now adjust the wavelength controls up and down and see how the color of the light changes. Page 4 of 10 GISAT 112 Fall 2008 6. If you increase or decrease the wavelength enough, you will not see any color because you will be beyond the region that your eye can detect. By careful observation, find out the maximum and minimum limits for your eyes and record these in your notes. 7. Finally, find out what wavelengths correspond to what colors. To do this, adjust the wavelength to 400 nm. Look down the tube and record on the data sheet (at the end of this document) the color you see. Then, adjust the wavelength to 440 nm and record the color you see in the data table. Repeat this at 40 nm intervals from 400 to 720 nm. You should have recorded a color corresponding to each wavelength you observed. Each student on your team should look at the light at each wavelength, and record his or her description of the color. B. Obtaining the Spectra of Red, Blue, Green, and Unknown Solutions 1. Investigate what four of the buttons on the instrument do. The “nm▲” and “▼nm” buttons will change the wavelength. The 0 ABS/100% T button is used to set the reading to 100 %T when the blank is in place. The A/T/C button switches among absorbance, % transmittance, and concentration modes. 2. Obtain 5 matched test tubes (called "sample cuvettes") that fit your spectrophotometer. Fill one cuvette about 2/3 full with pure water, your “blank”. Fill the remaining cuvettes 2/3 full with red, blue, green, and unknown solutions, respectively. These will be your "samples". 3. Place the "blank" tube in the instrument and close the cover. Set the wavelength to 400 nm and press the 0 ABS/100% T button so that the screen reads 100% T. 4. Now remove the "blank" tube, insert the "sample" tube with the red solution, and close the cover. Without pressing any other buttons, carefully observe the meter reading and record this in the data table as %T for the red solution at 400 nm. 5. Repeat step 4 for the blue, green, and unknown samples. (Optional: You can check the accuracy of the readings by repeating steps 2-4. Small fluctuations in the electrical voltage can sometimes cause fluctuations in the readings. Repeated measurements can catch this, but they will also make the whole process much slower and may not be needed for this study.) 6. Now change the wavelength to 420 nm. Repeat steps 3, 4, and 5 by first inserting the "blank," closing the cover, and pressing the 0 ABS/100% T button. Then insert the samples, observe the meter readings, and record the %T for each. 7. Proceed in the same fashion, at 20 nm intervals, all the way to 700 nm. 8. Examine the data to observe patterns. In what wavelength region did the solutions transmit the most light? In what wavelength region did they absorb the most light? Is this pattern consistent with the colors you observed in Part A? Page 5 of 10 GISAT 112 Fall 2008 C. Making Graphs of Your Results Use the graph paper provided to plot the data for each colored solution or substance. The wavelength should be on the horizontal axis (x axis), ranging from 400 nm at the left edge to 700 nm at the right edge. %T should be on the vertical axis (y axis) and range from 0 to 100. First plot the red solution. Carefully plot the data, making a small point with a circle around it for each measurement. When you are finished, draw a smoothly curved line through the points as best you can. The line may not touch every point. (This is better than "connect the dots," which is likely to produce a somewhat jagged line.) Repeat this procedure with other colored substances. If you are careful, you can plot more than one spectrum on the same graph. Label each spectrum so that you know which is which. For the unknown solution, convert your %T data to Absorbance (A): A = log (100/ %T) Plot your absorbance values on the Absorbance vs Wavelength graph provided. Identify the unknown by comparing your spectrum of the unknown solution with 5 spectra that will be supplied to you in lab. D. Extra Credit: Prepare a Standard Curve for the Blue Dye Solution 1. 2. 3. 4. Look at the %T spectrum you prepared for the blue dye solution. Select a wavelength that is absorbed strongly by this solution. Set the spectrometer to that wavelength (check the setting of the filter). Obtain %T readings for that solution and at least 4 dilutions of that solution with deionized water. Discuss your procedure with your instructor or a TA before you prepare the dilutions. 5. Convert your %T results to Absorbance values. 6. Plot Absorbance vs Concentration of the Dye. [For this exercise, arbitrarily assign the undiluted solution a concentration of 100 mg/L.] This is a standard curve that could be used by an analyst to measure the strength of solutions containing the dye you selected in step 1. LABORATORY REPORT The report for this experiment will be a full report. For guidance, refer to the the ISAT Style Manual for laboratory reports. The ISAT Style Manual is linked on the ISAT home page at: http://www.isat.jmu.edu/common/projects/StyleManual/ISATStyleManual.htm One report, jointly prepared by all members of your team, will be due in two weeks. The following questions are to be answered in the discussion section of your lab report. 1. For the red solution: a. Which region of the visible spectra has the highest transmittance? b. Which region has the lowest transmittance (and highest absorbance)? c. What visible color(s) (from your observations in Part A) correspond to the region of Page 6 of 10 GISAT 112 Fall 2008 low transmittance and high absorbance? 2. Similarly, for the blue solution: a. Which region of the visible spectra has the highest transmittance? b. Which region has the lowest transmittance (and highest absorbance)? c. What visible color(s) correspond to the region of low transmittance and high absorbance? 3. Whenever you see a clear red solution, what can you say about the color that is absorbed to produce the red color? 4. Which solution (red or blue) absorbs light of highest energy? Explain your reasoning. 5. Does your spectrum of the green solution look as you would predict? Explain briefly. 6. Describe the unknown sample and its spectrum. Is the spectrum approximately what you would predict? 7. What is the identity of the unknown sample? [Refer to the sheet of spectra that were supplied in lab.] 8. If you prepared a standard curve, include it in your report and discuss how such a curve might be used. Page 7 of 10 GISAT 112 Fall 2008 Data Sheet 1- Spectrometry Lab Wavelength (nm) Color Observed on Block 400 Page 8 of 10 GISAT 112 Fall 2008 Data Sheet 2- Spectrometry Lab Wavelength (nm) Red Solution (%T) Blue Solution Green Solution (%T) (%T) 400 Page 9 of 10 Unknown Sample (%T) A GISAT 112 Fall 2008 Percent Transmittance (%T) Graphs- Spectrometry Lab 100 80 60 40 20 0 400 450 500 550 600 650 700 Wavelength (nm) Absorbance 2.5 2 1.5 1 0.5 0 400 450 500 550 600 Wavelength (nm) Page 10 of 10 650 700