A1 :Distribution coefficient
advertisement
Questions
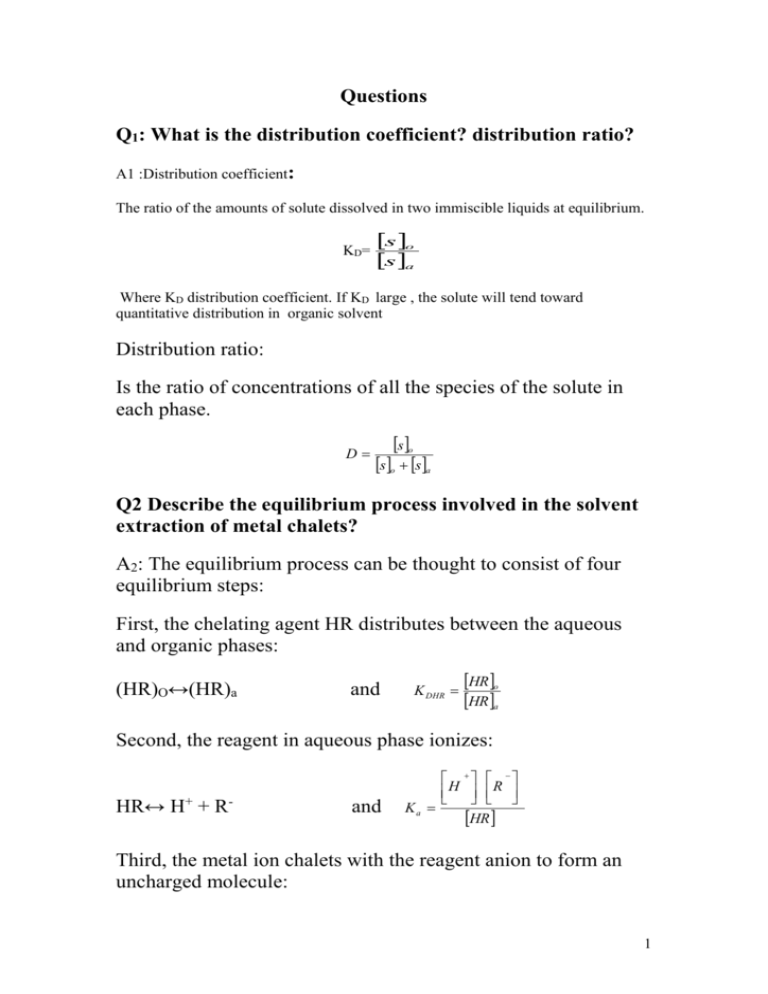
Q1: What is the distribution coefficient? distribution ratio?
A1 :Distribution coefficient:
The ratio of the amounts of solute dissolved in two immiscible liquids at equilibrium.
KD=
s o
s a
Where KD distribution coefficient. If KD large , the solute will tend toward
quantitative distribution in organic solvent
Distribution ratio:
Is the ratio of concentrations of all the species of the solute in
each phase.
D
s o
s o s a
Q2 Describe the equilibrium process involved in the solvent
extraction of metal chalets?
A2: The equilibrium process can be thought to consist of four
equilibrium steps:
First, the chelating agent HR distributes between the aqueous
and organic phases:
(HR)O↔(HR)a
and
K DHR
HR o
HR a
Second, the reagent in aqueous phase ionizes:
HR↔ H+ + R-
and
H R
Ka
HR
Third, the metal ion chalets with the reagent anion to form an
uncharged molecule:
1
M+ + nR-↔ MRn
and
Ka
NRn
M R
n
n
Finally, the chelate distributes between the organic and aqueous
phases
(MRn) ↔ (MRn)o
and
KD
MRn o
MRn a
KDHR and KDMRn are the distribution coefficients of the reagent
and the chelate, respectively; Ka is the ionization constant of the
regnant and Kf is the formation constant of chelate.
Q3 Describe two principle solvent extraction systems for
metal ions. give examples of each?
Two principle solvent extraction systems for metal ions:
1-Ion-Association complexes:
The metal ions is incorporated into a bulky molecule and then
associates with another ion of the opposite charge to form an ion
pair, or the metal ion associates with another ion of great
size(organic-like).
e.g.: Iron III can extract from HCl in to diethyl ether
{(C2O5)2 O: H+, FeCl4 (C2O5)2]-}
2-metal chalets
Metal ion is formation of chelate molecule with an organic
chelating agent. the cheats are often insoluble in water and will
precipitate. They are usually soluble in organic solvents.
e.g.: Diphenylthiocarbzone (dithizone), which forms a chelate
with lead ion.
2
Problems
11- ninety- six percent of solute is removed from 100 ml of an
aqueous solution by extraction with two 50 ml portions of an
organic solvent. What is the distribution ratio of the solute?
Va
E 1
D
V
V
o
a
n
100
96 1
50
D
100
2
100
96 1
50 D 100
2
100
95
50 D 100
9.75
2
100
50 D 100
487.34 D -975 = 100
D
100 975
487.34
D =2,21
3
12-the distribution ratio between 3 M HCl and tri-n-butyl phosphate for PbCl2 is 2.3
what percent PbCl2 will be extracted from 25.0 ml of 7.0×10-4 M solution into 10.0 ml
tri-n-butyl phosphate?
100 D
%E
V
D a
Vo
%E
100 2,3
25
2.3
10
%E
230
2.3 2.5
%E
230
4 .8
47.9 ≈ 48%
4
14-Ninety percent of metal chelate is extracted when equal volumes of aqueous and
organic phases are used> what will be the percent extracted if the volume of the
organic phase is doubled?
100 D
%E
D 1
90
100 D
D 1
90 D +90 = 100 D
90 = 100 D – 90 D
90+ 10 D
D
90
10
D= 9
If Vo =2 Va
100 D
Va
D
2Va
100 9
%E
1
9
2
900
%E
9.5
%E
94.7≈ 95%
5