Atoms, Ions, Isotopes
advertisement
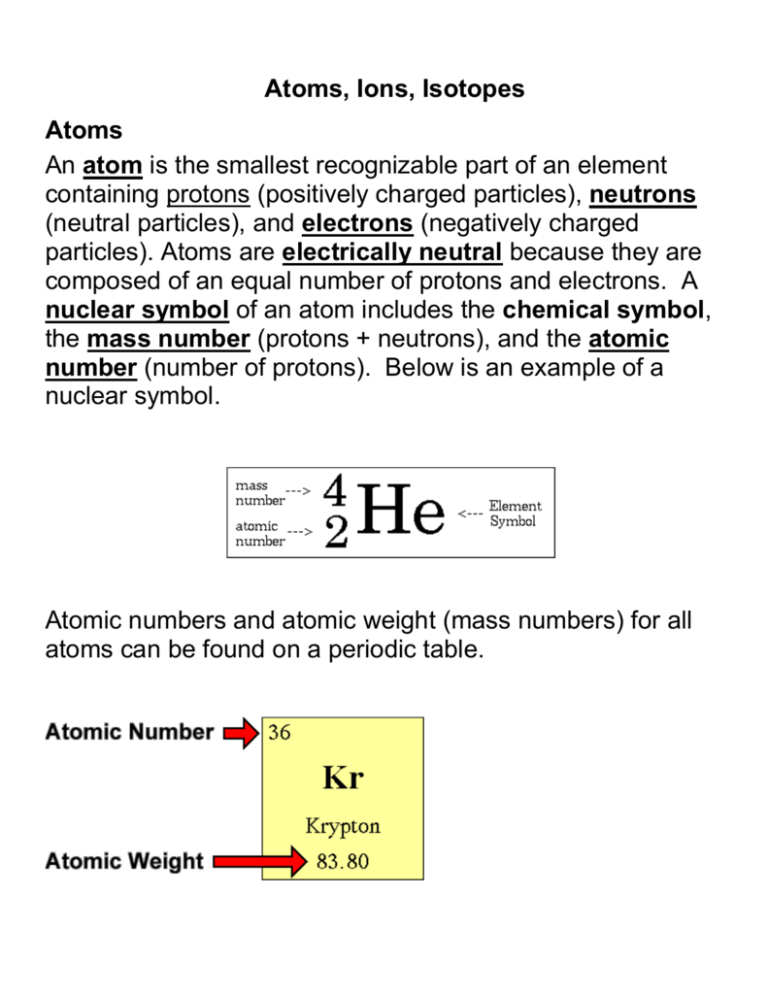
Atoms, Ions, Isotopes Atoms An atom is the smallest recognizable part of an element containing protons (positively charged particles), neutrons (neutral particles), and electrons (negatively charged particles). Atoms are electrically neutral because they are composed of an equal number of protons and electrons. A nuclear symbol of an atom includes the chemical symbol, the mass number (protons + neutrons), and the atomic number (number of protons). Below is an example of a nuclear symbol. Atomic numbers and atomic weight (mass numbers) for all atoms can be found on a periodic table. Ions An ion is an atom that has gained or lost electrons. In nonnuclear chemistry an atom never gains or loses a proton; only the number of electrons is affected during chemical reactions. The following shows how a hydrogen ion is formed. The image on the left is a hydrogen atom; therefore containing an equal number of protons and electrons. The atom on the right has lost its electron (usually to another atom during a chemical reaction); it is now an ion, and not an atom, because there is no longer an equal number of protons and electrons. The result is a charge imbalance known as a hydrogen ion. (H1+) Hydrogen Atom (H) Hydrogen Ion ( H1+ ) Now let's look at how a positive ion of beryllium is formed. Below, on the left, is a neutral atom of beryllium containing four protons and five neutrons. Circling the nucleus are four electrons. On the right, the atom has lost two electrons. The resulting ion still has four protons in the nucleus but contains only two electrons. There are now two more positive than negative charges; therefore the ion has an overall charge of 2+. (Be2+) Beryllium Atom ( Be ) Beryllium Ion ( Be 2+ ) A negative ion is formed in a similar way. Below, on the left, is an image of a neutral fluorine atom containing nine protons, nine electrons, and ten neutrons. When fluorine enters into a compound, it picks up an electron from the atom it bonds with. The resulting ion, called a fluoride ion, contains ten electrons and nine protons, with an overall charge of 1- (F1-) Isotopes Fluorine Atom (F) Fluoride Ion ( F1- ) Isotopes are atoms of the same element that contain different numbers of neutrons and thus have different masses. Below are drawings of the three naturally occurring isotopes of hydrogen; note that they differ only in the number of neutrons contained in their nucleus. The isotope in Fig. 1 contains only one particle in its nucleus: this isotope has a mass of one atomic mass unit and is known as protium. Fig. 2 shows a rare isotope of hydrogen. Its nucleus contains two particles (a proton and a neutron) and has a mass of two atomic mass units. It is known as deuterium. Figure 3 depicts an isotope known as tritium. The nucleus contains three particles (one proton and two neutrons) and has an overall mass of three atomic mass units. An isotope’s name is written using its element name and its mass. Look at Figures 1-3 below. Hydrogen – 1 Hydrogen – 2 Hydrogen - 3