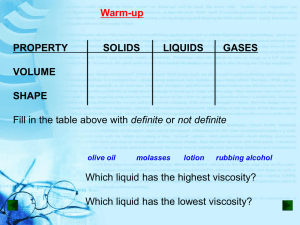
Boyle`s Gas Tube Experiment
advertisement

Boyle’s Gas Tube Experiment Robert Boyle (1627 – 1691) established the law that, for a given quantity of a gas (ideal) at a fixed temperature, the pressure is inversely proportional to the volume: P = A/V, where A is a parameter whose value is to be estimated from experimental measurements of pairs of values of P and V. By pouring mercury into the open top of the long side of a J-shaped tube, Boyle increased the pressure of the air trapped in the short leg. The volume of the trapped air would be V = h*cross section of short leg, where h is the height of the air in the short leg. If y = height of mercury in long leg – height of mercury in short leg, then we have Y = 0 + 1x, where x = 1/h. Due to the fact of experimental error, the model should be written as Yi = 0 + 1xi + i, where i = random error associated with ith measured pair of values of x and y. In his experiment, Boyle took 25 measurements of y at 25 specific different values of x. The data are shown below, together with the SAS program which analyzes the data. data one; input h y; x = 1/h; prod = h*y; label x = "Reciprocal of Height in Short End" y = "Height in Long End"; cards; 48 29.4375 46 30.5625 44 31.9375 42 33.5000 40 35.3125 38 37.0000 36 39.3125 34 41.6150 32 44.1875 30 47.0625 28 50.3125 26 54.3125 24 58.8125 23 61.3125 22 64.0625 21 67.0625 20 70.6875 19 74.1250 18 77.8750 17 82.7500 16 87.8750 15 93.0625 14 100.4375 13 107.8125 12 117.5625 ; proc print; Title "Data from Boyle's Gas Tube Experiment"; ; proc plot; plot y*(x prod); Title "Scatterplot of Height in Long End vs. Reciprocal of Height in Short End"; Title2 "And vs. the Product of the Heights"; ; proc corr; var h x y prod; Title "Correlations Among Variables"; Title2; ; proc reg; model y = x; plot predicted.*x; plot residual.*y; Title "Analysis of Boyle's Data on Gas Pressure vs. Volume"; ; run;