Gas Laws - Madison Public Schools
advertisement
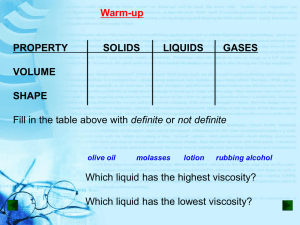
Physical Characteristics of Gases The Gas Laws Objectives • Students will be able to use Boyle’s Law to calculate volume and pressure changes at constant temperature. • Students will be able to use Charles’ Law to calculate volume and temperature changes at constant pressure. Class Warmup 1. A weather report gives the current atmospheric pressure reading of 745.8 mm Hg. Express this reading in the following units: a. atmospheres b. torr c. kilopascals 2. What will happen to the pressure of a gas if the volume of its container is decreases. Explain in terms of gas particles. Boyle’s Law • 1662 – Robert Boyle discovered the relationship between pressure and volume • The volume of a fixed amount of gas is inversely proportional to the pressure at constant temperature. Boyle’s Law Boyle’s Law: PV = k Or P1V1=P2V2 Sample Problems 1. A helium-filled balloon contains 125 mL of gas at a pressure of 0.974 atm. What volume will the gas occupy at standard pressure? 2. A weather balloon with a volume of 1.375 L is released from Earth’s surface at sea level. What volume will the balloon occupy at an altitude of 20.0 km, where the air pressure is 10.0 kPa? 3. The piston of an internal combustion engine compresses 450. mL of gas. The final pressure is 15 times greater than the initial pressure. What is the final volume of the gas, assuming constant temperature? Charles’ Law • The relationship between volume and temperature of a gas was discovered by Jacques Charles in 1787. • The volume of a fixed mass of gas at constant pressure is directly proportional to the Kelvin temperature. Charles’ Law • Charles Law: V k T Or V1 T1 V2 T2 Sample Problems Assume constant pressure for #’s 1-2. 1. A balloon filled with oxygen gas occupies a volume of 5.5 L at 25oC. What volume will the gas occupy at 100.oC? 2. A sample of nitrogen gas is contained in a piston with a freely moving cylinder. At 0.0oC, the volume of the gas is 375 mL. To what temperature must the gas be heated to occupy a volume of 500. mL? 3. An aerosol paint can has a warning label that advises against storage at high temperatures. What is the reason for this warning? Explain in terms of gas particles Gay-Lussac’s Law Question: What would happen if the temperature of a sample of gas in a rigid, sealed container was increased? Gas particles gain kinetic energy and move faster. This causes increased #’s of collisions and collisions with greater intensity. As a result, the pressure increases. Gay-Lussac’s Law • The relationship between pressure and temperature was recognized by Joseph Gay-Lussac in 1802 • The pressure of fixed amount of gas at constant volume is directly proportional to the Kelvin temperature. •Gay-Lussac’s Law P k T P1 T1 P2 T2 Sample Problems 1. An empty aerosol-spray can at room temperature (20oC) is thrown into an incinerator where the temperature is 500oC. If the gas inside the empty container was initially at a pressure of 1.0 atm, what pressure did it reach inside the incinerator? Assume the gas was at constant volume and the can did not explode. 2. The temperature within an automobile tire at the beginning of a long trip is 25oC. At the conclusion of the trip, the tire has a pressure of 1.80 atm. What is the final Celsius temperature within the tire if its original pressure was 1.75 atm? Next Question What happens when gas particles are added to a container that that is free to expand or contract. Assume the temperature is constant. Avogadro’s Law • Discovered in 1811 by Amadeo Avodgadro • Equal volumes of a gas at constant temperature and pressure contain equal number of particles. • At constant temperature and pressure the number of moles of gas is directly proportional to the volume. Avogadro’s Law V k n V1 n1 V2 n2 Sample Problems 1. 5.00 L of a gas is known to contain 0.965 mol. If the amount of gas is increased to 1.80 mol, what new volume will result (at an unchanged temperature and pressure)? 2. A cylinder with a movable piston contains 2.00 g of helium, He, at room temperature. More helium was added to the cylinder and the volume was adjusted so that the gas pressure remained the same. How many grams of helium were added to the cylinder if the volume was changed from 2.00 L to 2.70 L? (The temperature was held constant.) Combined Gas Law •In many situations, pressure, volume, and temperature all change. •Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law can be combined. •The combined gas law expresses the relationship between P, V, and T for a constant amount of gas PV k T P1V 1 T1 P2 V 2 T2 Sample Problems 1. The volume of a gas at 27.0oC and 0.200 atm is 80.0 mL. What volume will the same gas sample occupy at standard conditions of temperature and pressure? 2. A gas occupying 75 mL at standard conditions is heated to 17oC while the pressure is reduced to 0.97 atm. What is the new volume occupied by the gas? Dalton’s Law of Partial Pressures • The pressure of a mixture of gases is equal to the sum of the pressures of each individual gas. • Partial pressure- pressure of an individual gas in a gas mixture. PT= P1 + P2 + P3 +…. Problem Calculate the partial pressure in millimeters of mercury exerted by the four main gases in air at 760 mm Hg: nitrogen, oxygen, argon, carbon dioxide. Their abundance by volume is 78.08 %, 20.95 %, 0.934 %, and 0.035 %, respectively.