CH28 - Bama.ua.edu
advertisement

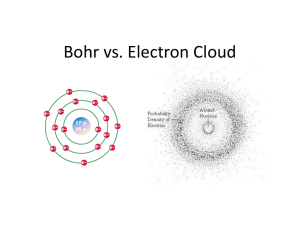
CH 28 – Atomic Physics Our present understanding of the atom is that it consists of a nucleus consisting of protons and neutrons with electrons in orbits about the nucleus. The neutron has no charge and the number of protons and electrons are the same so that the atom has no net charge. The diameter of the nucleus is much smaller than the diameter of the atom, but the nucleus contains most of the mass of the atom. This model of the atom was not accepted until the early part of the 1900s. An earlier model proposed by J.J. Thomson did not have a nucleus. Instead, it had electrons embedded in a cloud of positive charge. The model is sometimes called the ‘plum pudding model’, where the pudding was the positive charge and the plums the electrons. The nucleus was first discovered by Ernest Rutherford. He did an experiment in which alpha particles were projected against a thin metal foil and the deflection of the alpha particles by the foil was measured. The experiment showed that most of the alpha particles traveled through the foil with very little deflection. However, some of the alpha particles were deflected by large angles, and some were even deflected backwards. If the atoms were like a plum pudding, then the scattering would be more uniform and none would be expected to be completely deflected backwards. Rutherford’s interpretation was that the atom must have a massive, positively charged nucleus. Bohr Theory of the Hydrogen Atom If a tube is filled with a gas such as hydrogen, helium, or neon at low pressure and a high voltage is applied between two electrodes in the gas, then a current will be produced and the gas will emit light. If this light is examined using a prism or diffraction grating, it can be seen to consist of discrete wavelengths. These spectral lines are evidence of quantized energy levels in the atoms of the gas. The first successful quantum theory of the atom was developed by Niels Bohr to explain the hydrogen spectrum. The basic assumptions in the Bohr model of the hydrogen atom are as follows: 1. The electron orbits the proton under the influence of the Coulomb force between the two charges. This is much like the moon orbiting the earth under the influence of the gravitational force. 2. Certain orbits are stable and no radiation is emitted while the electron is in these orbits. (Classically, a charge moving in a circle would radiate electromagnetic energy.) 3. If an electron jumps from one orbit to another, the energy difference would appear as an emitted photon. That is, Ei E f hf (1) 1 4. The angular momentum of the orbiting electron can only have certain discrete values given by mvr n , n 1, 2,3, ... (2) where h / 2 . The above assumptions lead to quantized values for the energy and radius of orbit. The force on the electron is given by F k e2 m r2 v2 r (3) By multiplying each side of this equation by r/2, we get the kinetic energy 1 mv 2 2 ke2 2r (4) Combining this equation with the equation for the quantization of the angular momentum, we can algebraically solve for r and get rn n 2 2 mke2 , n 1, 2, 3, ... (5) Eq. (5) gives the allowed orbits of the electron. The lowest orbit obtained for n = 1 is called the Bohr radius ao and is ao 2 2 0.0529 nm (6) mke We can write the allowed radii in terms of the Bohr radius as rn n 2 a0 , n 1, 2, 3, ... (7) The four lowest radii are r1 a0 0.053 nm r2 4a0 0.21 nm r3 9a0 0.48 nm r4 16a0 0.85 nm 2 The energy of the electron is E KE PE 1 mv 2 2 e2 k r (8) Using the expression for KE from Eq. (4), we can write the energy as E ke2 ke2 ke2 2r r 2r (9) Using the value of radius from Eq. (5), we get En mk 2 e 4 1 , n 1, 2, 3, ... 2 2 n 2 (10) Eq. (10) gives the allowed values of the energy of the hydrogen atom. The negative sign means that the electron is bound the proton. If E 0, then the electron is unbound. Numerically, we can write the energy as En 13.6ev n2 , n 1, 2, 3, ... (11) The four lowest energy values are 13.6ev 13.6 ev 1 13.6ev 3.40 ev 4 13.6ev 1.51 ev 9 13.6ev 0.85 ev 16 E1 E2 E3 E4 The ground state energy (n = 1) is -13.6 ev. This means that it would take 13.6 ev to remove the electron from the atom. This is the ionization energy. 3 The allowed energies are shown in the energy level diagram below. When the atom is in an excited state (n > 1), it can make transitions to a lower level and lose its energy by emitting a photon with frequency f Ei E f h mk 2 e 4 1 1 4 3 n f 2 ni 2 The inverse wavelength is 1 where RH 1 f mk 2 e 4 1 1 1 RH , n 2 n 2 c 4 c 3 n f 2 ni 2 i f mk 2 e 4 4 c 3 1.097 x10 7 m 1 is the Rydberg constant. Conversely, a hydrogen atom in a lower energy level can absorb a photon and make a transition to a higher level if the energy of the photon is equal the energy difference between the two levels. 4 It is conventional to classify the emission lines according to the final state, nf, in the transition as shown in the energy level diagram. The series are given as follows. nf = 1: Lyman series nf = 2: Balmer series nf = 3: Paschen series etc. The emission spectra can be observed using a prism or grating spectrometer. Only the Balmer series would have wavelengths in the visible part of the spectrum. These visible lines would correspond to transitions from states 3 2, 4 2, 5 2, and 6 2. All other transitions would be in the ultraviolet or infrared region. The spectral lines for the Balmer series are shown below. The wavelengths of the measured spectral lines for hydrogen are in good agreement with those calculated by the Bohr theory. Example: Calculate the wavelength corresponding to the transition ni = 3 to nf = 2. Solution: 1 1 ( 1.097 x10 7 / m ) 2 2 32 1 6.56 x10 7 m 656 nm ( red ) 5 Example: What is the shortest wavelength in the emission spectrum of hydrogen? Solution: This would correspond to ni = to nf = 1. 1 1 1.097 x10 7 / m ( 1.097 x10 7 / m ) 2 2 1 91 nm ( uv ) 1 The Bohr Theory assumes one orbital electron. Of course, the only one-electron atom is hydrogen. However, the model would also apply to singly ionized helium, He+, doubly ionized lithium, Li++, etc., which also have only one orbital electron but for which the atomic number Z is greater than one. We would need to modify the model to account for the fact that the charge of the nucleus is Ze and replace e2 with (Ze)(e) = Ze2 in the Coulomb force and energy terms. This would then give the following for the energy, emission wavelength, and radius of orbit. En Z 2 ( 13.6 ev ) mk 2 Z 2 e 4 1 , n 1, 2, 3, ... 2 2 n 2 n2 mk 2 Z 2 e 4 1 1 , 4 c 3 n f 2 ni 2 1 rn n 2 2 mkZe2 n 2 a0 , n 1, 2, 3, ... Z Example: What is the energy required to remove the ‘last’ electron from lithium? Solution: For lithium, Z = 3. So E | E1 | Z 2 ( 13.6ev ) ( 3 )2 ( 13.6ev ) 122 ev 6 Example: What is the radius of orbit of doubly-ionized lithium? Solution: a 0.053nm r1 0 0.018 nm Z 3 De Broglie Waves and the Bohr Model One of the assumptions in the Bohr model is that angular momentum is quantized: mvr n , n 1, 2,3, ... This condition is equivalent to assuming that the orbiting electron is a wave with momentum p = h/ and that there are an integral number of wavelengths in one complete orbit. circumference 2 r n mvr nh nh p mv nh 2 Hydrogen Atom Wave Function and Spatial Quantum Numbers The Bohr Theory is very successful in predicting the energy levels of the hydrogen atom. It also suggests that the electron has a wave-like nature as described above. However, it does not completely describe other quantum effects and it does not sufficiently describe the wave nature of the electron. In addition, the Bohr Theory does not accurately describe the nature of multi-electron atoms. In 1926 Erwin Schrödinger published a paper in which he described a differential equation which could be used to solve for the wave function of the hydrogen atom. The physical meaning of is that its square gives the probability of finding the particle. Schrödinger’s equation is not limited to the hydrogen atom, but can in principle be used to determine the wave function of any physical system. In the case of the hydrogen atom, meaningful solutions for only occur for certain quantum numbers n, l, and ml. The quantum number n is called the principal quantum number, l is the orbital quantum number, and ml is the orbital magnetic quantum number. The allowed values of these ‘spatial’ quantum numbers are 7 n = 1, 2, 3, … l = 0, 1, 2, …, n-1 ml = - l, - l + 1, …, l - 1, l For each value of n, there are n allowed values of l, and for each value of l there are 2 l+1 allowed values of ml. Schrödinger’s equation predicts that the energy of the hydrogen atom is the same as given by the Bohr Theory, that is mk 2 e 4 1 En 2 2 n 2 It predicts that the magnitude of the angular momentum is L ( 1 ) and the component of the angular momentum along an axis is Lz m The angular momentum given by the Schrödinger equation is different from the Bohr Theory, which is L n . Also, in the Bohr Theory Lz is not quantized. Example: A hydrogen atom is in a state with principal quantum number n = 2. What are the allowed values of the magnitude and z-component of the angular momentum? Solution: For n = 2, the allowed values of l are 0 and 1. So, 0, m 0 : L 0(0 1) 0, L z (0) 0 1, m 0, 1 : L 1(1 1) 2, Lz 0, In the Bohr model, L n 2 and Lz could have any value between -2 and 2. Schrödinger’s equation does not predict discrete orbits, as in the Bohr Theory. Rather the wave function suggests that the electron can be envisioned as a ‘cloud’ surrounding the nucleus, with the density of the cloud giving the probability of finding the electron. The 8 electron cloud and the most probable location of the electron depend on the quantum numbers n, l, and ml. The electron cloud and the probability of finding the electron as a function of radius are given below for the lowest state (n, l, ml = 1, 0, 0). While the electron has a chance of being found anywhere, the most probable radius for the (1, 0, 0) state is the same as the Bohr radius. All states for which l = 0 have spherically symmetrical wave functions, somewhat like that shown above for n = 1, and their most probable radius is the same as given in the Bohr Theory; that is, r n 2 a0 . Those states for which l > 0 do not have spherical wave functions. Electron Spin Schrödinger’s equation predicts that the electron has an orbital angular momentum. In addition, the electron has an intrinsic ‘spin’ angular momentum. Although this is not a classical concept, the spin of the electron is analogous to the angular momentum of the earth as it rotates about its axis. The electron spin angular momentum has a fixed magnitude and can be measured either ‘up’ or ‘down’. The up or down component has the value ½ . In general, we can write S z m s , m s 1 2 Allowed Quantum States of the Hydrogen Atom For each set of spatial quantum numbers n, l, ml we can have ms = ½. The first 28 allowed states are summarized in the following table 9 n 1 2 3 l 0 0 1 0 1 2 ml 0 0 -1,0,1 0 -1,0,1 -2,-1,0,1,2 ms ½ ½ ½ ½ ½ ½ no. l 2 2 6 2 6 10 no. n 2 total no. 2 8 10 18 28 Shell and Subshell Notation Those states which have the same principal quantum number n are said to lie in the same ‘shell’. The shells corresponding to n = 1, 2, 3, 4, … are referred to as the K, L, M, N, …shells. So, there are 2 states in the K shell, 8 states in the L, shell, 18 states in the M shell, etc. Those states which have the same orbital quantum number l are said to lie in the same ‘subshell’. The subshells corresponding to l = 0, 1, 2, 3, … are referred to as s, p, d, f, …. There are 2 states in the s subshell, 6 states in the p subshell, 10 states in the d subshell, etc. Shorthand notation for describing a specific subshell corresponding to a given values of n and l is as follows: n, l = 1, 0: = 2, 0: = 2, 1: = 3, 0: = 3, 1: = 3, 2: etc. 1s 2s 2p 3s 3p 3d Multi-electron Atoms In principle the Schrödinger equation can also be used to solve for the wave function, energies, and angular momentum of atoms that contain more than one electron. The problem is much more complex than for hydrogen because of the interactions of the electrons with each other. These interactions include both Coulomb interactions and magnetic interactions. The magnetic interactions occur since there are magnetic moments associated with the orbital and spin angular momentum of the electrons. These interactions give different energy values than would be obtained by treating each electron independently. However, it is found that the states of the electrons can still be described using the four quantum numbers (n, l, ml, ms). 10 The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of quantum numbers. So, we could imagine building up a multi-electron atom by filling up the available states given in the table above in order of increasing energy. So, in the ‘ground’ state, the two electrons in helium would have the quantum numbers (n, l, ml, ms) = (1, 0, 0, ½) and (n, l, ml, ms) = (1, 0, 0, -½). We describe the ‘electronic configuration’ of helium as 2s2, where the superscript refers to the number of electrons which occupy the 2s subshell. Likewise, for lithium, we would have (n, l, ml, ms) = (1, 0, 0, ½), (1, 0, 0, -½), and (2, 0, 0, ½), and the electronic configuration would be 1s22s1. The table below gives the electronic configurations of the elements up through Kr. The states are filled in order of increasing quantum number through Ar. The outer electron for K, however, is 4s1 instead of 3d1. This is because the interactions between electrons cause the 4s1 state to have lower energy than the 3d1 state. There are other departures in the filling sequence for higher Z numbers for a similar reason. Z 1 2 3 4 5 6 7 8 9 10 11 12 Sym bol H He Li Be B C N O F Ne Na Mg Elect. Conf. 1s1 1s2 [He]2s1 2s2 2s22p1 2s22p2 2s22p3 2s22p4 2s22p5 2s22p6 [Ne]3s1 3s2 Z 13 14 15 16 17 18 19 20 21 22 23 24 Sym bol Al Si P S Cl Ar K Ca Sc Ti V Cr Elect. Conf. 3s23p1 3s23p2 3s23p3 3s23p4 3s23p5 3s23p6 [Ar]4s1 4s2 3d4s2 3d24s2 3d34s2 3d54s1 Z 25 26 27 28 29 30 31 32 33 34 35 36 Sym bol Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Elect. Conf. 3d54s2 3d64s2 3d74s2 3d84s2 3d104s1 3d104s2 3d104s24p1 3d104s24p2 3d104s24p3 3d104s24p4 3d104s24p5 3d104s24p6 Energy Levels of Multi-electron Atoms The calculation of the energy levels of multi-electron atoms is complex because of the interactions between the electrons. However, in some cases the Bohr model can be used to estimate the energy levels. One example would be the excited states of the Alkali metals such as Na or K. The electronic configuration of Na, for example, is 1s22s22p63s1. The first 10 electrons form closed shells like that of Ne, and the 3s1 valence electron is loosely bound compared to the inner electrons. When the 3s1 electron is in an excited state, then it ‘sees’ a spherically symmetric charge distribution consisting of a nucleus with charge +11e shielded by an electronic charge distribution with charge -10e. Thus, the effective nuclear charge is +e, the same as for hydrogen. Indeed, the energy levels of Na in the excited states are similar to that of hydrogen in its excited states. 11 Similarly, a core electron, say, in a K shell (n = 1), ‘sees’ a nuclear charge +Ze that is shielded by approximately one electronic charge –e due to the other electron in the shell. So, the effective nuclear charge is Zeff = (Z-1)e. Thus, the energy of the K electrons can be estimated from the Bohr model to give En E1 Z 2 ( 13.6 ev ) n2 Z eff 2 ( 13.6 ev ) (1 ) 2 ( Z 1 ) 2 ( 13.6 ev ) Example: Estimate the energy of a K shell electron in Cu. Solution: For Cu, Z = 29. So E1 (29 1) 2 (13.6 ev) 10,662 ev So, the energy required to extract a core electron from Cu is huge compared to the energy to ionize hydrogen. X-rays are produced in x-ray tubes when high-energy electrons strike a metal target in the tube. One of the sources of the x-rays results from electronic transitions between core levels. The high energy electron can penetrate the core of the atom and dislodge one of the inner core electrons. Then an electron in an upper level makes a transition to this lower level and loses its energy in the form of a high energy photon - an x-ray. Example: What is the approximate energy of an electron in the M (n = 3) shell of Cu when there is a vacancy in the K shell? Solution: The M shell electron would be shielded by one electron in the K shell and 8 electrons in the L shell. So, the effective nuclear charge would be Zeff e = (Z-9)e. Thus, for Cu E3 ( 29 9 ) 2 ( 13.6 ev ) ( 3 )2 ( 20 ) 2 ( 13.6 ev ) 604 ev 9 12 Example: What would be the wavelength of the photon if an electron in Cu makes a transition between the M shell and a vacancy in the K shell? Solution: From the above, E E3 E1 604 ( 10662 ) 10058 ev hc hc ( 6.6 x10 34 J s )( 3x108 m / w ) 1.23 m 0.123 nm E ( 10058ev )( 1.6 x10 19 ev / J ) Energy Levels in Solids If atoms are brought together to form a solid, then the interactions between the atoms causes the discrete electronic energy levels of the atoms to split into closely spaces levels grouped together in bands. The number of levels in a band is of the order of the number of atoms in the solid, so the energy levels in a band are so closely spaced as to be nearly continuous. In general, the bands are separated by energy gaps. The figure below illustrates this splitting of the 1s and 2s levels as two, five, and then a very large number of atoms are brought together. 13 The nature of the upper most bands determines whether a solid is a conductor, an insulator, or a semiconductor. In order for a solid to conduct, there must be empty states into which the electrons can move. If the upper band is partly filled, then there are empty states which can be easily accessed and the solid is a conductor. A partially filled band will exist if a filled band overlaps with an empty band. If all bands are either completely filled or completely empty, then the solid may be either an insulator or a semiconductor, depending on the energy gap Eg between the filled valence band and the empty conduction band. If Eg is sufficiently small, then the solid will be a semiconductor. In this case, electrons in the valence band can be thermally activated to occupy levels in the bottom of the conduction band, leaving empty states (holes) in the top of the valence band. Conduction can then take place in both the conduction band and in the valence band. The electron conduction in the valence band is equivalent to the holes moving in a direction opposite to the conduction electrons. The holes behave as positive electronic charges. Common semiconductors are silicon and germanium. For silicon, Eg = 1.1 ev and for germanium Eg = 0.66 ev. If the energy gap is very large, then no thermal activation of electrons from the valence band into the conduction band can take place and the solid is an insulator. Diamond is a very good insulator and has an energy gap of about 5.5 ev. 14