Lecture 1.14 Density
advertisement

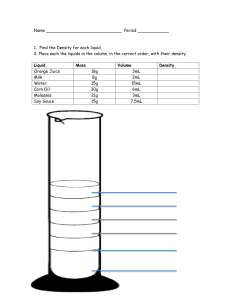
Page 1/2 Lecture 1.14 Density Density = Mass / Volume Density Units: g/mL or g/cm3 or g/cc Objective: Understand the facts about density: 1. The density of a given mass of a substance is temperature dependent. Therefore, changes in temp will cause changes in density. 2. Density is a convenient conversion factor when you need a specific mass of a liquid. For example, it is difficult to weigh out 15mL of liquid B. It is much more precise to measure out 15mL of liquid B using a graduated cylinder. Calculate the mass using algebra if the density of liquid B is .987g/mL. DxV=M .987g/mL x 15mL = 14.80 or 15g Why does ice float when it is a solid and water is a liquid? Shouldn’t it be more dense and sink? Objective: Practice Density Calculations: 1. Milk has a density of 1.03 g/mL at room temp. Calculate the mass of 1L of milk. density = mass/volume So mass = density x volume Convert 1L to mL: volume = 1L x 1000mL/L = 1000mL mass = 1.03g/mL x 1000mL = 1030g or 1.03 x 10 3g (=1.030kg) 2. A diamond has a density of 3.5 g/cm3. Calculate the volume of 0.5g of diamond. density = mass/volume So, volume = mass/density volume = 0.5g/3.5g/cm3 = 0.14cm3 Homework Problems: 1. Blood cells have a mean cell volume of 9.0 x 10 -14 L. Is this an appropriate unit to measure the volume of a blood cell? Why or why not? Convert this to cc, then calculate the density of a 5 gram blood sample. (9.0 x 10 -14 L/1) (1000cc/1L) = 9.0 x 10 -11cc D = M/V 5g/9.0 x 10 -11 cc D = 5.6 x 10 10 g/cc Next Page Find Go to Page Thumbnail Index Image View Download a Copy Close