Chem Math - Fredericksburg City Public Schools
advertisement

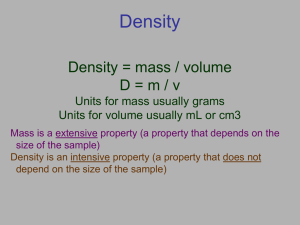
Chemistry is where you learn 2 + 2 = 10 or something. —Dennis Rodman Chemistry Math With Chemistry-specific graphs Variables Independent Variable “I” change Graphed on “x” axis Dependent Variable which “Depends” on independent variable Tested during experiment Graphed on “y” axis Control Variables you control so they do not change and mess up your experiment Never graphed Graphing Variables Slope = rise/run Slope = mass/volume Slope = density We don’t graph just to graph; the graph and the data tells us something meaningful. Line of Best Fit Real data never (almost never) falls exactly on the line! The line is an “average” Once you know the line of best fit, you can use it to predict other values. The line represents the data. We never “connect the dots” in a graph. Don’t connect the dots. The line of best fit doesn’t have to go through ANY of the data points. However, some points might be exactly on the line. Ideally the points above the line = the points below the line y = mx + b To graph without your calculator, you need 2 points to define a line. Use the y-intercept for the first point (x = 0 when y = b) Set y = 0 to find the second point (x = − b/m when y = 0). Use a RULER to draw your lines! Helpful Formulas Unit 1 Density D = m/V Temperature Conversions K = °C + 273 (note K does not have a ° symbol) °C = K − 273 °C = (°F − 32) ÷ 1.8 ΔT = T2 − T1 or ΔT = Tfinal − Tinitial Directly Proportional Directly proportional means that as one goes up, the other goes up too. For matter, kinetic energy (KE) is directly proportional to Kelvin temperature (K) If you graph these variables, you will get a straight line with a POSITIVE slope. Inversely Proportional Inversely proportional means that as one goes up, the other goes down (and vice versa). For any gas, pressure (P) is inversely proportional to volume (V) If you graph these variables, you will get a straight line with a NEGATIVE slope. Let’s Do Some Math! Density If a marble weighs 10.0 g and has a volume of 5.0 mL what is the density of the marble? Use correct units. Another marble with the same density has a mass of 15.0 g. What is the volume of that marble? Use correct units. A different marble has a density of 3.0 g/mL. If that marble has a volume of 10.0 mL, what is the mass of that marble? Use correct units. Let’s Do Some Math! Temperature If it is 104°F in Fredericksburg, what is the temp in °C? What is the temp in K? If it is -40°F in Fredericksburg, what is the temp in °C? What is the temp in K? If a sample of matter is 298K, what is the temp in °C? Helpful Formulas Unit 1 How to Figure out your Quiz Grade: Your Grade ÷ 25 × 100 = SCORE Calculating Error Error = |Your Number − True Number| % Error = Error ÷ True Number × 100 Let’s Do Some Math! Error Analysis The accepted value for the density of a penny is 7 g/cm3. You measure 5 pennies and calculate the density as 7.50 g/cm3 7.20 g/cm3 6.90 g/cm3 7.40 g/cm3 6.90 g/cm3 What is the average density? What is the error? What is the percent error? Let’s Do Some Math! What is the average density? 7.18 g/cm3. What is the error? |7.18 − 7| = 0.18 g/cm3. What is the percent error? 0.18 ÷ 7 x 100 = 2.57142857 You should record your final answer as 2.6% after rounding. Helpful Formulas Unit 1 SI Units and Conversions Need to know what these prefixes mean Kilo (k) Centi (c) Milli (m) Micro (μ) Nano (n) ex 1 kg = 1000 g ex 100 cm = 1 m ex 1,000 mL = 1 L ex 1,000,000 μg = 1 g ex 1,000,000,000 nm = 1 m Dimensional Analysis The base unit can change, but the prefix means the same thing 100 cm = 1 m 100 cg = 1 g 100 cL = 1 L Centi ALWAYS means that there are 100 divisions of the base unit. Let’s Do Some Math! Convert 45 cm to mm? To do this you need to know the conversion factors 100 cm = 1 m 1,000 mm = 1 m. First step: convert cm to meters. Second step: convert meters to mm. To find the answer: cancel out units until you have the units you are looking for. Let’s Do Some Math! Convert 45 cm to mm? 45 cm × 1m 100 cm 45 cm × 1m 100 cm × 1,000 mm = ? 1m × 1,000 mm = 1m 450 mm Let’s Do Some Math! Convert 345 μg to kg? Convert 14.6 mL to L? Convert 1 hour to seconds? Convert 100 cm3 to mL Convert 500 nm to mm? Let’s Do Some Math! Answers: Convert 345 μg to kg? 3.45 x 10-7 kg Convert 14.6 mL to L? 0.0146 L Convert 1 hour to seconds? 3,600 sec Convert 100 cm3 to mL 100 mL Convert 500 nm to mm? 5 x 10-4 mm Let’s Do Some Math! Answers: Convert 345 μg to kg? 3.45 x 10-7 kg Convert 14.6 mL to L? 0.0146 L Convert 1 hour to seconds? 3,600 sec Convert 100 cm3 to mL 100 mL Convert 500 nm to mm? 5 x 10-4 mm Some Other Chemistry Graphs Phase Diagrams Heating Curves Radioactive Decay Reaction Progress Not all chemistry graphs will be straight lines. But most will. Let’s see some distinctive ones which aren’t. Phase Diagram for H2O Critical Point Triple Point A phase is a “state” of matter. Solid phase Liquid phase Gas phase We don’t “do” plasma. Phase Diagram Critical Point Triple Point The triple point is the only point on the graph where all 3 phases are at equilibrium. The critical point is the END of the graph. Phase Diagram Boiling Point Melting Point The lines show the pressure/temperature points where the phase changes from one to the other. When P = 1 atm, you can read the MP and BP right off the graph. Heating Curve for H2O Solid between A&B, liquid between C&D, gas between E&F Heating Curve for H2O Melts between B & C Boils between D & E Why doesn’t the temp rise when water is melting? Radioactive Decay After 1 half life, 50% remains. After 2 half lives, 25% remains. HALF of whatever is left decays during each half life. Rxn = abbreviation for reaction Rxn Progress The reaction is exothermic (products have less energy than reactants). Enzyme = biological catalyst. Rxn Progress This reaction is endothermic. Products are higher than reactants (have more potential energy). The End