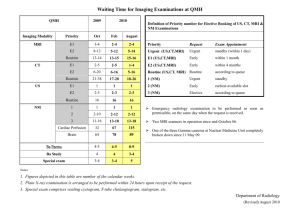
The MagnaSafe Registry
advertisement

Determining the Risks of MRI at 1.5-Tesla for Patients with Pacemakers and Implantable Cardioverter Defibrillators (The MagnaSafe Registry) Author Block: Jennifer Cohen, Heather Costa, Robert Russo, Scripps Clinic, La Jolla, CA Abstract: Background: The MagnaSafe Registry is a multi-center study designed to evaluate the risk of MRI at 1.5-Tesla for 1500 patients with pacemakers and implantable cardioverter-defibrillators (ICD) who undergo clinically-indicated non-thoracic imaging. Methods: Device interrogation was performed pre- and post-MRI using a standardized protocol. For pacemaker-dependent patients the device was programmed to an asynchronous pacing mode, and pacing functions were deactivated for non-dependent patients. For non-pacemaker dependent ICD patients, all tachyarrhythmia therapies were disabled, while pacemaker dependent ICD patients were excluded. Noninvasive monitoring was performed throughout the MRI, and a physician proficient in device programming was present. Primary endpoints were death, device failure, generator/lead replacement, induced arrhythmia, loss of capture, or electrical reset. Secondary endpoints were clinically relevant device parameter changes: Results: Between April 2009 and March 2010, 105 clinically-indicated MRI studies were performed at 3 sites. Mean patient age was 73 ± 14 yrs. Seventy-four pacemakers and 31 ICDs with 206 leads were scanned. Pacemaker dependence was noted in 21% (22/105). MRI duration was 38 ± 19 min and was performed an average of 3.0 ± 1.7 yrs after device implant. Pacer dependence was noted in 21% (22/105). There were no deaths, device failures, generator/lead replacements, induced arrhythmias, losses of capture, or electrical reset. One episode of selfterminating atrial fibrillation was noted. A decrease in battery voltage of ≥0.04 V occurred in 2%of devices, a pacing lead impedance change of ≥50 Ω in 3%, and a high voltage impedance change of ≥3 Ω in 10%. A decrease in pacing threshold P and R wave amplitude occurred in 0% and 2%, respectively. A pacing threshold increase of ≥ 0.5V at 0.4ms occurred in 1% (2/185) of leads. Twelve percent of MRI studies resulted in at least one parameter change. Conclusions: Preliminary results of the MagnaSafe Registry demonstrate no deaths, device failures, generator/lead replacements, induced arrhythmias, losses of capture, or electrical reset episode, and a low rate of clinically relevant device parameter changes after clinically-indicated non-thoracic MRI at 1.5 Tesla.