Laboratory report:Diffusion and Osmosis
advertisement

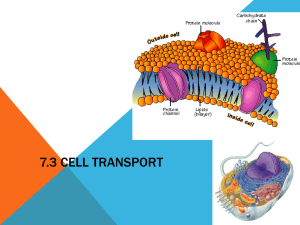
Daiva Kuncaite SCB 201 Prof. Wejesinghe 10/06/09 Week III Title: Diffusion and Osmosis. Objectives: to describe the mechanism and importance of diffusion and osmosis. Define selective permeable membrane and explain its role in osmosis. Define hypotonic, hypertonic and isotonic solutions and how the cells behave in each of the solution. Explain the influence of the cell wall on osmotic behavior in the cell. Introduction: In order to maintain the homeostasis in the cell the substances inside the cell has to move out of the cell and substances presented in the extracellular fluids must move in. Therefore, the movement of the substances can be achieved by different transport methods. Diffusion is a net movement of particles from an area of high concentration, to the area of low concentration. Particles move by random motion and using kinetic energy. It occurs due to Brownian motion and concentration gradient. Selectively permeable membrane allows only small substances with low molecular weight can pass through by diffusion. Osmosis is a diffusion of water through a selectively permeable membrane from its region of high concentration to its region of low concentration. All cells must maintain their osmotic environment and water uptake. Animal cells are very sensitive to the osmotic pressure and placed in the hypotonic solution the cell membrane undergo lysis, and when it placed in the hypertonic solution, the cells shrink or crenate. The plant cell, on the other hand, due to the structure of cell wall will not burst or crenate. Procedure: In the first experiment we observe diffusion due to Brownian movement. On clean slide we put one drop of water. We dipped a tip of a dissecting needle in the dry carmine dye and added on the drop of water on the slide. Covered with watch glass and observed the movement of the particles under the microscope. In the second experiment we determine the permeability of assigned chemical substances using dialysis tubing, which is an artificial semi permeable membrane. We took dry dialysis tubing and soaked in the water for few minutes. One end of the tube we tide with the string. In the tube we added 4 pipettesful of 30 % glucose solution and 4 pipettesful of starch solution. We tide the opening of the tube with the string that made solution will not spill. Meantime we prepared a 500 ml beaker with 300 ml of water with several drops of I2KI solution until the water become yellow-amber color. We placed the bag in the solution and left on the table for 30 min. After 30 min. we observed the changes in both solutions. In order to determine the presence of glucose molecules we performed Benedict’s test. We took 3 test tubes. In first test tube we took a sample of 2 pipettesful from the dialysis bag, in the second test tube – a sample of 2 pipettesful from the beaker and the third sample was with water. In each test tube we added 1 drop of Benedict’s reagent and heated the test tubes in the hot water bath for about 3 min. We recorded our observations. Daiva Kuncaite SCB 201 Prof. Wejesinghe 10/06/09 Week III In the third experiment we investigated the osmotic behavior of a plant cells placed in different molar solutions. We took a small sample of the Elodea leaf in distilled water solution and placed on the slide with the drop of water. We cover it with watch glass and observe the cells under the microscope. On the second slide we took a sample of Elodea leaf in a NaCl (hypertonic) solution. We placed the watch glass and observe the cells under the microscope. Results and Conclusions: In the first experiment we observed the movement of carmine particles in water. Since the particles were moving randomly, bouncing to each other we can conclude that we actually observed molecular movement. The movement never stopped. The smaller particles were moving more rapidly than the large ones. The movement we observed was driven by the kinetic energy of the molecules. The intrinsic molecular kinetic energy is the driving force of the diffusion. Diffusion is important in cells life, because there are a lot of chemical reactions that takes place inside the cell. Some reactions need oxygen which comes by the diffusion. Carbon dioxide, as a waste, also leaves the cell by the diffusion. In the second experiment we made a hypothesis that substances that have small molecular structure will pass easily thorough the dialysis tubing and relatively large molecules will not pass through the dialysis tubing. Our prediction was that Iodine molecules diffuse into the bag and glucose molecules will diffuse to the beaker. Starch, as large polysaccharide will remain in the bag. As a result, the color of the bag from cloudy white turned blue black, which indicated that iodine molecules have diffused into the bag. The bag color started changing the moment we placed it in the beaker because iodine molecules started diffusing into the bag. Therefore we concluded that iodine molecules are very small. After performed Benedict’s test the sample from the bag turned yellow. We concluded that not all glucose molecules had diffused through the dialysis membrane and the remains of the glucose molecules showed positive Benedict’s test results. The sample from the beaker after Benedict’s test turned orange, which indicated the presence of glucose. Therefore, glucose had diffused through the dialysis membrane. Our prediction and hypothesis was confirmed. In the third experiment: Elodea in distilled water solution Elodea in NaCl (hypertonic) solution We observed the cell of the Elodea leaf in distilled water solution that contained central vacuole and the chloroplasts against the cell wall. The water is hypotonic solution toward the cell, but the Daiva Kuncaite SCB 201 Prof. Wejesinghe 10/06/09 Week III cell wall made out of the cellulose prevents of the excess intake of water into the cell. It exerts osmotic pressure that opposes water movement to the plant cell and prevents it from bursting. The cell of Elodea leaf in NaCl solution looked much different. The cell wall was visible and intact, but inner organelles of the cell shrank. That indicated that cell was placed in hypertonic solution and water came out from the cell. However, the cell wall remained intact and only plasma membrane detached from the wall and shrank. It is different from the animal cells, because animal cell don’t have cell walls made out of cellulose, therefore the cells crenate placed in the hypertonic solution. We try to reverse the process in the plant cell by soaking Elodea leaf in the water for 10 min. and that observing if any changes occurred. We noticed that plasma membrane expend a little bit and chloroplast dispersed. That concluded that reverse process in the plant cells also can occur by placing the cells back to the hypotonic solution. The pond water is hypotonic to the Elodea cells. The cell wall show rigidity and resistance to slight ionic gradient. Homework: 1. Describe plant wilting in terms of turgor pressure. When water moves into plant cells by osmosis it makes the cell expand. However, the cell walls are very strong and cannot expand much. So the pressure rises inside the cell – it becomes tightly filled it reaches the greatest turgor pressure. This makes the cells rigid and helps to support plant’s leaves. If the plant looses too much water or runs short of water in the soil, the plant will die because of the water loss. 2. The emergency room intern, Jim W. Dooley, treated a patient by administering fluids intravenously. The patient died as a result of using the wrong IV fluids. What kind of osmotic solution would have resulted in the patient’s death? Why? The administration of hypotonic or hypertonic solution would have resulted in the patient’s death. Human cells placed in hypotonic solution will undergo lysis and cells placed in hypertonic solution will undergo crenation. 3. Tori, an ER volunteer and amateur botanist, is shocked by the death of Dooley’s patient. The next time she waters her flower garden she wonders why applying pure water does not kill plants, but makes them thrive? Pure water for the plans is hypotonic to the cell, however, the turgor pressure and presence of cell wall made out of cellulose prevents of the excess intake of water into the cell. It exerts osmotic pressure that opposes water movement to the plant cell and prevents it from bursting. Daiva Kuncaite SCB 201 Prof. Wejesinghe 10/06/09 Week III 4.The pond water samples you observed probably contained a variety of multi-cellular, colonial, and single-celled organisms. Some of these may have had cell walls, but others were lacking cell walls. What adaptation for osmoregulation is found in single-celled organisms, such as the Amoeba, and multi-cellular organisms that lack cell wall but live in hypotonic environment? These organism have contracting vacuole that gather the excess water and expel it to the outside of the cell.