MEASUREMENTS: LENGTH, MASS AND VOLUME
advertisement

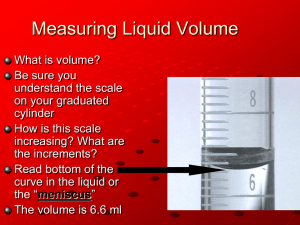
Experiment 2: Measurements As part of their daily work, scientists must carry out common laboratory procedures, take measurements, and report their results accurately and clearly. The system of measurement used in modern science is the metric system. The metric system is a decimal-based system, meaning that measurements of each type are related by factors of 10. The metric system has one standard unit for each type of measurement: the standard unit for length measurement is the meter (m), the unit for mass measurement is the gram (g), and the unit for volume is the liter (L). When taking measurements of quantities that are significantly larger or smaller than the standard unit, prefixes are attached in front of the standard unit. The most common prefixes are list below. Prefix kilo deci centi milli Symbol k d c m Meaning 1000 1/10 (0.1) 1/100 (0.01) 1/1000 (0.001) PROCEDURE A. Measuring Length a. Observe the marked lines on a meter stick. Identify the lines that represent centimeters and millimeters. Record your observations. Observations (include a drawing, and label the length represented by each line): b. Estimate the length and width of this paper in centimeters, and record your estimate here: Estimated length:__________cm width: __________cm . Now, use the meter stick or a ruler to measure the length and width of this paper in cm. Estimate the last digit if it fells between the lines. Actual length:__________cm width: __________cm c. Using a piece of string and the meter stick, measure the length of your shoes in centimeters. Length of shoes: __________cm Page 2-1 B. Measuring Volume a. Remove a test tube from your drawer (do not use the wider test tubes). Fill the tube with DI water to the rim (almost overflowing). Hold your large (100 mL) graduated cylinder next to the test tube. Estimate the volume of water in this test tube in milliliters (mL) using the graduated cylinder for comparison. Estimated volume _________mL Carefully pour the water into the large graduated cylinder (use your dropper to adjust the volume to the last line). Record the volume. Actual volume _________mL (using 100 mL graduated cylinder) b. Fill the test tube with DI water to the rim again. Pour the water into a medium (50 mL) sized graduated cylinder. Record the volume reading. Actual volume _________mL (using 50 mL graduated cylinder) c. Repeat this process using a small (10 mL) graduated cylinder. You must do this in several steps, by first transferring no more than 10 mL of water to the graduated cylinder at a time, and then pouring the water into the sink. When you are done, obtain the volume by adding the total volume measured. Tip:pour about 9 mL of water into the cylinder, then use your dropper to transfer enough water to exactly match the 10 mL line. This will give you the most accurate results. Actual volume _________mL (using 10 mL graduated cylinder) (Calculations) d. Obtain a plastic gallon jug and a large 1L graduated cylinder from the cart. Fill the graduated cylinder with tap water to the 1 Liter mark. Estimate how many liters are in one gallon, and write down this value. Estimated volume _________L (your estimate does not need to be a whole number!) e. Carefully pour the water from the graduated cylinder into the gallon jug. Continue to refill the graduated cylinder with water and pour water into the gallon jug until a total of one gallon of water has been added. From this, determine how many liters are in a gallon. Have the instructor initial your results. Actual volume _________L Instructor Initial: ___________ Page 2-2 C. Measuring Mass a. After your instructor shows you how to use a laboratory balance, determine the mass of a 50mL beaker, a stopper (on cart), and a metal disk (also on the cart). Copy the number or letter on the disc on your report sheet (if there is one) You must always record all of the digits from the balance reading on your report sheet. Do not round off or drop any zeros! Mass of beaker: __________g Mass of stopper: __________g Mass of disc# : __________g b. Pour just enough rock salt into the beaker you weighed in the previous step to cover the bottom of the beaker. Then, estimate the mass and write down your estimation. Estimated mass of salt: __________g Use the balance to determine the mass of your salt, and record the value. Mass of salt + beaker: __________g Calculations Mass of salt: __________g Instructor’s Initial:_______ c. Set the salt aside (perhaps on the watchglass or a piece of paper towel) for use in part D. d. Pour about 1 g of sugar into the same beaker. Estimate and measure the mass of the sugar, just as you did in part b. Estimated mass of sugar: __________g Mass of sugar + beaker: __________g Mass of sugar: __________g Page 2-3 D. Measuring Temperature a. Determine the temperature of the room by simply reading the value on your thermometer. Note that if the temperature reading appears to be “on the line”, you should write .0 at the end of the measurement; if it is between the lines you should write .5 Temperature of room: _________oC b. Fill your 250-mL beaker about half way to the top with deionized (DI) water. Then, record the temperature of the water. Temperature of water: _________oC c. Put a handful of ice into the beaker, and stir it for about a minute with your glass stirring rod (never stir with your thermometer!) Then, record the temperature of the slush you have made. Temperature of ice-water: _________oC d. Pour the salt from Section C into the ice water, and stir it for about two minutes. Record the temperature of this mixture. Temperature of ice water: with salt _________oC Copy all values from these sheets over to the Report Sheet. Do not forget to copy all units! You will only turn in the Report Sheet when you are done. Save the remainder to help you review for the lab final. Page 2-4 Report Sheet Name:________________________ MEASUREMENTS 1. Measuring Length a. What units are represented by the numbers marked on the meterstick? ________________ There are ____________ centimeters in 1 meter. There are _____________ millimeters in 1 cm. There are ____________ millimeters in 1 m. b. Estimated value Measurement Length of the paper Width of the paper c. Length of your shoe __________________ 2. Measuring Volume Estimated volume of the test tube _______________________ Largest cylinder Medium cylinder Smallest cylinder Size of the cylinder (in mL) Volume of water from the test tube Estimated volume of one gallon of water __________________________ Actual volume of one gallon of water ________________________ C. Measuring Mass a. mass of 50-mL beaker __________________ b. mass of the stopper ____________________ c. mass of the disk ___________________ code # of the disk __________________ Page 2-5 d. Estimated value Measurement 5 g of salt 1 g of sugar D. Measuring Temperature a. Temperature of the air in the room________________ b. Temperature of the water_______________ c. Temperature of the ice-water_________________ d. Temperature of the salt/ice-water mixture______________________ Be sure that you have included the correct units for all answers, where appropriate! Questions . 1. Balances can be used to weigh objects of many different masses and sizes. Why would a balance at a truck-stop which is used to weigh 18-wheelers and their cargo be inappropriate for determining your own weight? Be as specific as possible. Page 2-6 2. Devise a way to measure the approximate thickness of a single sheet of paper in millimeters, using a ruler and paper (and maybe a calculator) as your only tools. (Only suggest a procedure; you do not need to carry it out.) Then, list the steps you would use clearly and in order. Page 2-7 Page 2-8 Materials for this experiment: Meter sticks String Size 4 stoppers Unknown metal disks 5 one-gallon plastic bottle salt 5 g/ student sugar 10 g per student 5 one-liter graduated cylinder ice Page 2-9