Estimating G values for transition state barriers
advertisement
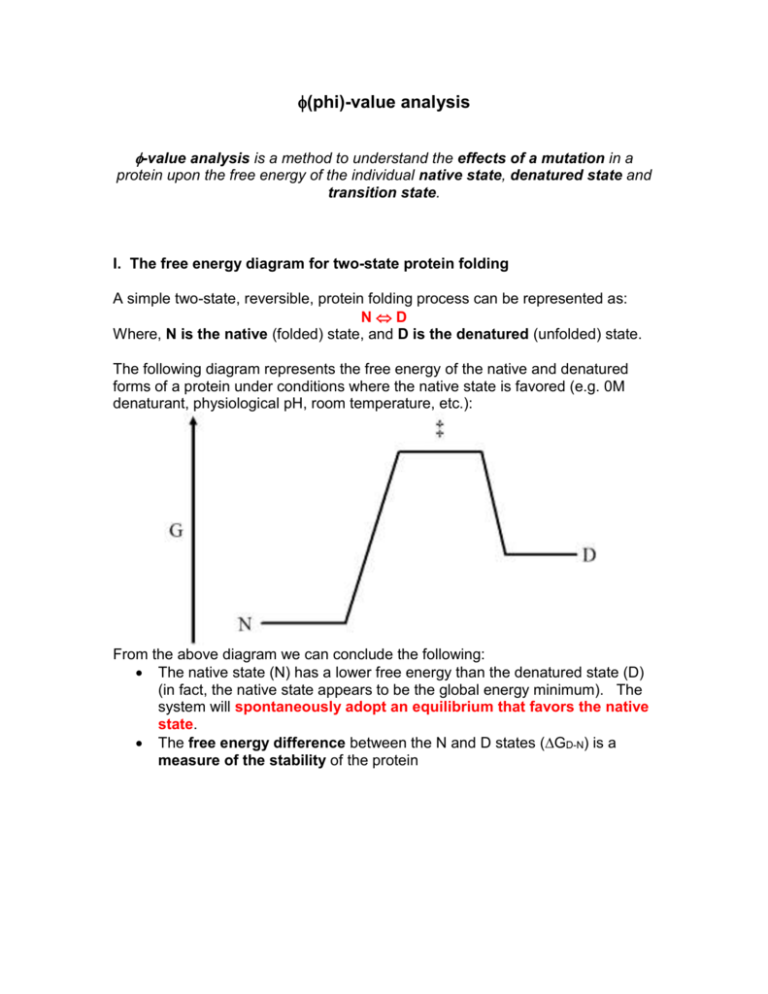
(phi)-value analysis -value analysis is a method to understand the effects of a mutation in a protein upon the free energy of the individual native state, denatured state and transition state. I. The free energy diagram for two-state protein folding A simple two-state, reversible, protein folding process can be represented as: ND Where, N is the native (folded) state, and D is the denatured (unfolded) state. The following diagram represents the free energy of the native and denatured forms of a protein under conditions where the native state is favored (e.g. 0M denaturant, physiological pH, room temperature, etc.): From the above diagram we can conclude the following: The native state (N) has a lower free energy than the denatured state (D) (in fact, the native state appears to be the global energy minimum). The system will spontaneously adopt an equilibrium that favors the native state. The free energy difference between the N and D states (GD-N) is a measure of the stability of the protein In this case, GND is defined as the free energy change in going from state 1 (N) to state 2 (D). "Delta" values are always defined as (value of state2 - value of state1), thus GND = (GD - GN) or GD - N From the above diagram this value should be a positive value A positive value indicates a non-spontaneous process as written (i.e. going to the right). Thus, N D is non-spontaneous in the right-hand direction, but the reverse direction is spontaneous as written (thus, the above diagram reflects a situation where the equilibrium favors the native state of the protein) II. Rates of folding and unfolding and the free energy diagram The folding transition state, ‡ , (which is actually likely to represent an ensemble of structures) describes the energy barrier between the N and D states, and determines the rate at which the N state converts to D (unfolding process), and the rate at which the D state converts to N (folding process) The energy barrier for unfolding is proportional to the height between the N‡ states The energy barrier for folding is proportional to the height between the D‡ states The rate at which system can change states is inversely proportional to the height of the energy barrier (i.e. the larger the energy barrier, the fewer molecules in the sample that will have the necessary energy to overcome the barrier, and therefore, the slower the overall rate of change of state) In the above diagram, the N‡ energy barrier (G‡ - N) is larger than the D‡ energy barrier (G‡ - D), thus: The rate of unfolding should be slower than the rate of folding This situation results in an equilibrium condition that will favor the N state (i.e. the above diagram is representative of a protein that is stably folded) The rates of folding and unfolding are a function of the rate constants for folding (kf) and unfolding (ku), thus, we have an overall diagram that looks like this: III. Equilibrium denaturation methods Equilibrium denaturation experiments report the extent of denaturation as a function of added denaturant (isothermal equlibrium denaturation by guanidine or urea), or added heat (differential scanning calorimetry). Such experiments provide information on the equilibrium constant for denaturation, and therefore, GD - N: G° = -RTln(Keq) These experiments assume the system is always at equilibrium (e.g. samples are allowed to come to equilibration prior to measurement), thus, they provide no information on the folding or unfolding kinetics (i.e. folding or unfolding rate constants) Thus, using these methods alone, we cannot say what the effects of mutations are upon the kinetic properties Example I: The mutant protein free energy diagram is shown in the green broken line, and the free energy of the native, denatured and transition states is indicated by an asterisk. The mutation has affected the transition state. In particular, it has stabilized the transition state and has had no effect upon either the native or denatured states In stabilizing (i.e. lowering the free energy of) the transition state, the mutation will result in an increase in both the rate of unfolding and folding However, since the free energy levels of the native and denatured states are unchanged, the overall GD - N value is unchanged. Thus, equilibrium denaturation methodologies would report no difference between the mutant and wild type proteins, but, kinetic experiments would clearly indicate that the mutation has altered the rates of folding and unfolding. -value analysis compares the free energy data from equilibrium denaturation methodologies to free energy values derived from kinetic studies, and this comparison allows a determination of how a mutation has affected the free energy of the native, denatured or transition states of the protein IV. Probing the structure of the transition state Example II: A mutation (indicated by an asterisk *) that does not affect the denatured state, or the transition state, but destabilizes the native state: In this example: The values of the folding rate constants, kf and k*f, for wild-type and mutant are observed to be equal, therefore, G‡ - D and G*‡ - D are identical in value and therefore the value of G‡ - D = 0 (i.e. G‡ - D - G*‡ - D = 0) Note: it may seem that when you make a mutation that the mutant should be considered the "new state" (i.e. state 2) in comparison to the wild type "previous state" (i.e. state 1). Thus, any delta values relating a mutant to wild type should be of the form: (mutant value - wild type value). However, there is no strict adherence to this frame of reference, and effects of mutations are commonly calculated by subtracting mutant values from the wild type. The key thing is that you explicitly state how you are calculating the values for the mutant in terms of the wild type protein when you report the relevant delta values. The unfolding rate constants are different between mutant and wildtype (faster for the mutant). Thus, G‡ - N values are different and the value of G‡ - N (i.e. G‡ - N - G*‡ - N) is non-zero (positive in this case). The value of G‡ - N can be determined from the wild type and mutant folding rate constants: k G‡ N RT ln u* ku (Note: G‡ - N is also referred to as Gunfolding or Gu) The GD - N value for the mutant (i.e. the effect of the mutation upon stability) is determined experimentally using isothermal equilibrium denaturation data (at the same temperature as the kinetic studies, or using DSC data with G value determined by extrapolation of individual G values to the temperature used for the kinetic experiments). Note that in the above case, the GD - N value is equal to the value of G‡ - N. In other words, it looks like if you make a mutation that affects the stability of the protein, and if this effect is characterized by changes upon the folding rate constants only, then the mutation has affected primarily the native state. G‡ N 1.0 then it means that the energetic changes between the wild type If GD N and mutant native states accounts for the entire energetic difference observed in the equilibrium stability study (and we conclude that the mutation has affected the native state exclusively) If a mutation affects the native state and transition states equally, then it is assumed that the mutation site is as folded in the transition state as it is in the native state (i.e. the mutation site adopts the native configuration in the transition state) If a mutation affects the denatured state and transition states equally, then it is assumed that the mutation site is as unfolded in the transition state as it is in the denatured state (i.e. the mutation site adopts the denatured configuration in the transition state) In the above example, the transition state and the denatured state are unaffected by the mutation - in other words, the mutation has affected these states equally - thus, we would conclude that the site of mutation is as unfolded in the transition state as it is in the denatured state In this case, the perturbation of the mutation upon the denatured state is equivalent to the perturbation of the transition state. Thus the site at this position is presumably unfolded in the transition state. Example III: In this example: The values of the unfolding rate constants, ku and k*u, for wild-type and mutant are observed to be equal, therefore, the values of G‡ - N and for G*‡ - N are identical and the value of G‡ - N = 0 The folding rate constants are different between mutant and wild-type (faster for the wild-type). Thus, G‡ - D values are different and the value of G‡ - D is negative. k G‡ D RT ln *f k f (Note: G‡ - D is also referred to as Gfolding or Gf) Note that in the above case, the GD - N value is equal to the value of G‡ - D. In other words, it looks like if you make a mutation that affects the stability of the protein, and if this effect is characterized by changes upon the unfolding rate constants only, then the mutation has affected exclusively the denatured state. G‡ D 1.0 then it means that the energetic changes between the wild type If GD N and mutant denatured states accounts for the entire energetic difference observed in the equilibrium stability study (and we conclude that the mutation has affected the denatured state exclusively) For this example, the perturbation of the mutation on the transition state is equivalent to the perturbation upon the denatured state. Therefore, the site of mutation is as unfolded in the transition state as it is in the denatured state. V. value analysis The basis of value analysis is to compare the overall free energy change for a mutation to the individual contributions of the folding and unfolding free energy change. The analysis usually is focused upon understanding whether a particular mutation site is folded or unfolded in the transition state (and in this way probes the "structure" of the transition state). The GD - N value is determined by denaturation equilibrium methods k u The Gu RT ln * value is determined from the unfolding kinetic ku constants of the mutant and wild type kf The G f RT ln * value is determined from the folding kinetic kf constants of the mutant and wild type G f is called the "folding value". If it equals 1.0 it means that F G D- N the site of the mutation is native-like in the transition state (a value of 0 means the opposite) Gu is called the "unfolding value". If it equals 1.0 it means U G D- N that the site of the mutation is denatured in the transition state (a value of 0 means the opposite) Fractional , or negative, values for values are more difficult to interpret Usually the choice of either f or u is based upon whether folding kinetic data or unfolding kinetic data can be more accurately determined. VI. Cross-validation GD - N values can also be determined from the kinetic values: GD N G‡-N G‡-D ku k * f GD N RT ln k * k u f If the two-state model correctly describes the protein denaturation, then the above value should agree with the value from equilibrium denaturation studies The above equation also suggests that If unfolding kinetic data for a mutant can be obtained, but folding kinetic data cannot, it can be predicted by comparing the equilibrium G data and the known kinetic data: GD N G‡-D G‡-N and G‡-N GD N G‡-D VII. Derivations The rate of folding is proportional to the free energy difference between the denatured state and the transition state: Assumptions: = 1.0 k *T b h Boltzmann's constant, kb = 1.380 x 10-23 J K-1 Temperature in K Planck's constant, h = 6.626 x 10-34 J sec has units of (J K-1)*(K)/(J sec) = sec-1 (appropriate for a rate constant) kf RT ln G‡-D Calculation of G‡-N from experimental rate constants of unfolding: Assuming and are the same in both cases: G‡-D follows a similar derivation.