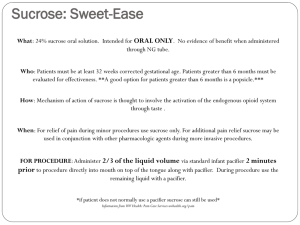
Guidelines for the Administration of 24% Sucrose
advertisement

Original Approval: June 2009 Type: Guideline Revised/Reviewed: 11/2010 Key Words: Oral Sucrose, Sucrose Analgesia, 24% Sucrose To be reviewed: 11/2013 Supersedes: None Nursing Services I. Title: Sucrose Analgesia: Guidelines for the Administration of 24% Sucrose Solution to Infants II. Purpose To provide guidelines for the safe administration of 24% sucrose oral solution to provide pain relief for infants. III. Applicability All UMHHC infants 25 weeks post conceptual age (PGA) up to six (6) months of age for pain relief and comfort related to procedural, acute and ongoing pain. . IV. Definitions Procedural pain indications for sucrose include but are not limited to Nasogastric/Oral Gastric/Nasal insertion Intramuscular or Subcutaneous injections peripheral/central/arterial line placement chest tube placement or removal invasive line removal arterial or venous puncture endotracheal suctioning suprapubic tap Retinopathy of Prematurity exam dorsal penile nerve block (circumcision) heel sticks tape removal lumbar puncture Continuous Positive Airway Pressure removal of pacing wires chest physiotherapy suture placement or removal Electrocardiogram V. Procedures/Actions A. Infants who are able to take sucrose orally or a pacifier coated with sucrose and would benefit from the therapy are screened by the RN for contraindications and precautions (see section for Contraindications and Precautions). B. Twenty-four percent (24%) sucrose can be ordered by an RN and administered by an RN, LPN, nursing intern/student, phlebotomist, medical assistant, respiratory therapist or parent. Page 1 of 6 Sucrose Analgesia: Guidelines for the Administration of 24% Sucrose Solution to Infants C. Sucrose and non-nutritive sucking can also be used to provide comfort with the management of acute and ongoing pain in infants. D. For maximum effect, sucrose should be administered at least 2 minutes prior to a painful procedure. When used for procedures, analgesic effect last approximately 5 minutes. Dose can be repeated every 2-3 minutes if needed. (Lefrak et al. 2006). E. Sucrose can be given as an adjuvant non-pharmacological therapy to promote comfort when an infant is experiencing acute pain or discomfort originating from other unknown sources of discomfort. F. Sucrose solution is not intended to be a substitute for appropriate analgesia. Use additional analgesics as indicated by the anticipated painfulness of the procedure and/or the infant’s response to the procedure. G. Administer sucrose directly from single patient use vial to the anterior part of tongue or coat pacifier with sucrose and offer to infant. H. Offer pacifier as tolerated/desired to encourage non-nutritive sucking to promote soothing and comfort. I. Dosage Guidelines: administer in 1 gtt increments as needed/as tolerated. Weight or Age Suggested dose per event <1000 gms: 1000-2000 gms: >2000 gms: Term infant and under 6 mos 0.05ml – 0.1 ml per event 0.05 ml – 0.2 ml per event 0.05-0.5 ml per event 0.05 ml -0.5 ml in increments up to 2 ml per event See Exhibit A for recommended dosing and procurement of the current Sucrose product J. There is no maximum number of doses however the need for additional analgesic intervention(s) should be assessed if more than 8-12 doses per day are administered. K. Document each dose (gtts or number of pacifier dips) and infant’s response on the patient care flow sheet. L. Monitor the infant’s pain responses using appropriate pain assessment tool during and after procedure, and ongoing. M. Family education should include the differentiation between sucrose analgesia, comfort and feeding. N. Contraindications 1. Sucrose is contraindicated in the following: a. Infants with active necrotizing enterocolitis (NEC). Page 2 of 6 Sucrose Analgesia: Guidelines for the Administration of 24% Sucrose Solution to Infants b. Infants in shock or with a recent history of low blood pressure/low perfusion state; infants believed to have significantly diminished mesenteric perfusion. 2. Precautions with sucrose administration should be taken with the following infants. a. Those at risk for NEC and who are being treated for suspected NEC. b. < 27 weeks PGA and those with cardiorespiratory instability (LeFrak, 2006). c. Infants with esophageal atresia, tracheal esophageal fistula, or any other GI surgical anomaly. 3. Literature has reported occasional transient desaturation with sucrose administration; this may be related to the volume administered and methods of administration. Use precaution in administering repeat doses if desaturation occurs. Desaturation may be reduced by using minimal volume, and by administering with a pacifier. (Lefrak, 2006). VI. Exhibits A. B. Dosage Guidelines Parent Information Sheet, PDF, Information for Parents: Oral Sucrose for Infants Having Painful Medical Procedures VII. References A. References: Harrison, D M, Oral sucrose for pain management in infants: Myths and misconceptions. Journal of Neonatal Nursing, 2008; 39-46. Harrison D, Stevens B, Bueno M, Ymada, J et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child 2010: 95:406-413. Hatfiled L A, Gusic, M E, Dyer, A and Polomano, R C. Analgesic properties of oral sucrose during routine immunizations at 2 and 4 months of age. Pediatrics 2008: 121e327-2334. Lefrak l., Burch, K., et al. Sucrose analgesia: Identifying potentially better practices, Pediatrics, 2006;118 (Supplement 2):197-202. Mitchell A, Waltzman P. Oral sucrose and pain relief for preterm infants. Pain Management Nursing, 2003: 4(2): 62-69. Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. The Cochrane Database of Systemic Reviews 2004, Issue 3. Page 3 of 6 Sucrose Analgesia: Guidelines for the Administration of 24% Sucrose Solution to Infants Johnston CC, et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks’ postconceptional age. Pediatrics. , 2002: 10(3): 523-528. B. Search Terms: Oral Sucrose, Sucrose Analgesia VIII. Related Policies/Guidelines/Standards/Procedures A. Anand KJS, et al. Consensus statement for the prevention and management of pain in the newborn. Archives Pediatric Adolescent Medicine, 2001; 155:173-180. IX. Author(s), Consultant(s) A. Authors: Wendy Kenyon BSN, RN, Neonatal Intensive Care Unit, Holden Jessie Antanaitis RN, Cardio-Thoracic Surgery Pediatrics Denise Spicer BSN, RN-BC, Acute Care Pediatrics, 5W Connie Ritter, AD, RN, Neonatal Intensive Care Unit, Holden Mary Watson RN, BSN, MSBA, Clinical Project Manager, Ambulatory Care Sandra Merkel MS, RN-BC, Clinical Nurse Specialist, Pediatric Acute Pain Service B. Consultants 1. Pediatric Medical-Surgical Joint Practice Committee Approved/Endorsed guideline June 19, 2009. 2. Pediatric Clinical Nurse Practice Network, October 14, 2010 X. Reviewed and Approved by: Integrated Clinical Council, 11/2010 Nursing Executive Council, 12/2010 Original guideline documents are held by the Nursing Integrated Clinical Council. Direct questions to NurseICC_Administration@med.umich.edu. Page 4 of 6 Sucrose Analgesia: Guidelines for the Administration of 24% Sucrose Solution to Infants Exhibit A Dosage Guidelines using Toot Sweet™ Twist-Tip vials Administer 1 gtt at a time as needed and as tolerated Weight or Age Suggested dose per event <1000 gms: 1000-2000 gms: >2000 gms: Term infant and under 6 mos 0.05ml – 0.1 ml per event (1-2 gtts) 0.05 ml – 0.2 ml per event (1-4 gtts) 0.05-0.5 ml per event 1-10 gtts) 0.05 ml -0.5 ml in increments up to 2 ml per event (2 vials) 1 ml vial = ~ 20 gtts Each drop = ~ 40 ul (microliters) To obtain Sucrose: TootSweet (24% Sucrose) Material Service Center (MSC) Item # = 43844 Solution, Sucrose TootSweet 1 ml Hawaii Medical Manufacturer # = 333.1040027BX Page 5 of 6 Sucrose Analgesia: Guidelines for the Administration of 24% Sucrose Solution to Infants Exhibit B “Information for Parents: Oral Sucrose for Infants Having Painful Medical Procedures”. Parent/Caregiver handout can be downloaded and printed http://www.med.umich.edu/i/nursing/policies/pediatrics/sucroseExB.pdf Page 6 of 6