Supplementary Information (doc 34K)
advertisement

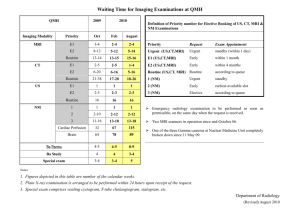
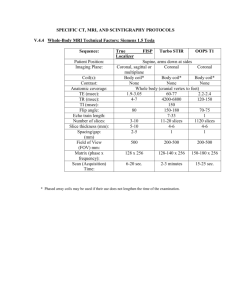
Supplementary Materials and Methods In vivo MRI In vivo brain status was assessed on a 4.7T horizontal bore MR system (Agilent, Santa Clara, CA) with use of a Helmholtz volume coil (90 mm diameter) and an inductively coupled surface coil (25 mm diameter) for signal excitation and detection, respectively. Anesthesia was induced with 5% isoflurane for endotracheal intubation and tail vein cannulation. During MRI, isoflurane was discontinued and anesthesia was maintained with 60 mg/kg/h propofol (20 mg/ml) i.v. following a 15 mg/kg bolus. Animals were ventilated with a mixture of O2/air (1:7). Blood oxygen saturation and heart rate were monitored using a pulse oximeter (8600V, Nonin Medical, Plymouth, MN), and end-tidal CO2 with a capnograph (Multinex 4200, Datascope Corporation, Paramus, NJ). Body temperature was maintained at 37.0 ± 0.5 °C. Anatomical images were obtained with a 3D gradient-echo (GE) sequence (repetition time (TR) / echo time (TE) = 6 / 2.25 ms; 40° flip angle; 4 averages; 256 × 128 × 128 matrix; field of view (FOV) = 60 × 40 × 40 mm). 10 minutes of resting-state fMRI was performed with a T2*-weighted single-shot GE-EPI sequence (TR / TE = 600 / 25 ms; 50° flip angle; 64 × 64 matrix; FOV = 32 × 32 mm; 13 × 1.5 mm coronal slices; 1000 images). To assess whether long-term methylphenidate exposure induced sustained alterations to dopaminergic neuronal signaling, pharmacological MRI with a dopamine-releasing agent was performed using a four-shot GE-EPI sequence (TR / TE = 1000 / 25 ms; 65° flip angle; 64 × 64 matrix; FOV = 32 × 32 mm; 19 × 1.0 mm coronal slices; 675 images) with 10 minute baseline scanning, and 35 minutes scanning following intravenous injection of 0 (vehicle; n = 3 per group) or 1 mg/kg (n = 13 per group) D-amphetamine in saline (1 mg/ml). At this dose, D-amphetamine has been shown to induce robust hemodynamic activation responses (Schwarz et al, 2004) that correlate with extracellular dopamine levels (Ren et al, 2009). Immediately after MRI, animals were euthanized with pentobarbital (300 mg/kg, i.p.) and transcardially perfused with cold phosphate buffered saline (PBS) followed by 4% formaldehyde in PBS. After overnight post-fixation in the skull, the brains were removed and stored in PBS with sodium azide. One animal died during transition to propofol, and four were excluded from the pharmacological MRI analysis because of physiological instability or complications during preparation. Postmortem MRI Dissected brains (n = 12 per group) were measured on a 9.4T horizontal bore MR system equipped with a 90 mm-diameter 1000 mT∙m-1 gradient coil (Agilent, Santa Clara, CA), using a transmit and receive birdcage coil (Millipede, Agilent, Santa Clara, CA). Brains were placed in a custom-made holder and immersed in a non-magnetic oil (Fomblin, Solvay Solexis, Weesp, The Netherlands). Anatomical images with an isotropic voxel size of 93.75 μm were acquired (3D GE; TR / TE = 6.87 / 3.34 ms; 15° flip angle; 64 averages; 320 × 160 × 200 matrix; FOV = 30 × 15 × 18.75 mm). DTI was performed to assess white matter status, using a diffusion-weighted eight-shot spin-echo EPI sequence (TR / TE = 2700 / 28 ms; 128 × 128 matrix; FOV = 25 × 25 mm; 55 × 0.2 mm transversal slices; 8 averages; b = 3174 s/mm2, δ = 5 ms, Δ = 11 ms; two sets of 60 diffusion-weighted images in non-collinear directions, and 6 unweighted images (b = 0)). Immunohistochemistry In a subset of the brains of experimental animals (n = 7-8 per group) 2′,3′-cyclic nucleotide 3′phosphodiesterase (CNPase) immunohistochemistry was used to quantify myelin content in the corpus callosum, anterior commissure, and striatum (at 2.28, 1.32, 0.60, and -0.60 mm relative to bregma). To this end, the fixed brains were cryoprotected by saturation in 30% sucrose in phosphate buffer (PB, 0.1M, pH 7.4) after which they were coronally sectioned in a one-in-ten series at 40 μm on a freezing microtome (R. Jung AG, Heidelberg, Germany). After sectioning, the series were washed twice in PB to get rid of remaining sucrose and were subsequently stored in PB with 0.01% sodium azide at 4ºC until further use. Standard horse radish peroxidase based diaminobenzidine immunohistochemistry was applied using a monoclonal mouse anti-CNPase antibody (clone SMI-91, Calbiochem). Before the staining procedure started, sections were mounted first on Superfrost slides. Slides were washed in PB and dipped into distilled water to get rid of buffer salts before incubating into methanol absolute for 5 minutes to unmask the antigen. After washing, endogenous peroxidase activity was blocked for 20 minutes by incubating in 0.5% hydrogen peroxide in PB and washed again with PB before blocking a-specific antigen sites for 60 minutes with 10% normal goat serum (NGS), 1% bovine serum-albumin (BSA) and 0.1% Triton X-100 (TX-100) in PB. The primary antibody incubation was performed in 5% NGS, 1% BSA and 0.1% TX-100 at a dilution of 1:7000 for 1 hour at room temperature and overnight at 4ºC. The next day, sections were washed with PB before incubating for 2 hours in sheep anti-mouse biotinylated secondary antibody (GE Healthcare) at a dilution of 1:200 in PB. After washing, peroxidase-labeled avidin biotin complexes (Vectastain Elite, Vector Labs) were incubated at a 1:800 dilution for 2 hours. The sections were once more washed in PB, followed by two washes in Tris-HCl buffer (TB, 0.05M, pH 7.6). Staining reaction was performed for 14 minutes using 3,3′-diaminobenzidine tetra-hydrochloride and hydrogen peroxide as substrates, and the reaction was terminated by 3 washes in TB. The slides were dehydrated in a graded alcohol series, cleared in xylene and coverslipped using Entallan. References Ren J, Xu H, Choi J-K, Jenkins BG, Chen YI (2009). Dopaminergic response to graded dopamine concentration elicited by four amphetamine doses. Synapse 63: 764–772. Schwarz A, Gozzi A, Reese T, Bertani S, Crestan V, Hagan J, et al (2004). Selective dopamine D(3) receptor antagonist SB-277011-A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse 54: 1–10.