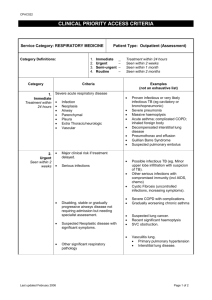
Respiratory failure often is divided into two main types
advertisement

Franc Šifrer Bolnišnica Golnik SEVERE RESPIRATORY FAILURE Respiratory failure is a syndrome in which the respiratory system fails in one or both of its gas exchange functions: oxygenation and carbon dioxide elimination. In practice, respiratory failure is defined as a PaO2 value of less than 60 mm Hg while breathing air or a PaCO2 of more than 50 mm Hg. Furthermore, respiratory failure may be acute or chronic. While acute respiratory failure is characterized by life-threatening derangements in arterial blood gases and acid-base status, the manifestations of chronic respiratory failure are less dramatic and may not be as readily apparent. In respiratory failure, the level of oxygen in the blood becomes dangerously low, and/or the level of C02 becomes dangerously high. There are two ways in which this can happen. Either the process by which oxygen and C02 are exchanged between the blood and the air spaces of the lungs (a process called "gas exchange") breaks down, or the movement of air in and out of the lungs (ventilation) does not take place properly. Hypoxemic respiratory failure (type I) is characterized by a PaO2 of less than 60 mm Hg with a normal or low PaCO2. This is the most common form of respiratory failure, and it can be associated with virtually all acute diseases of the lung, which generally involve fluid filling or collapse of alveolar units. Some examples of type I respiratory failure are cardiogenic or noncardiogenic pulmonary edema, pneumonia, and pulmonary hemorrhage (1). Hypercapnic respiratory failure (type II) is characterized by a PaCO2 of more than 50 mm Hg. Hypoxemia is common in patients with hypercapnic respiratory failure who are breathing room air. The pH depends on the level of bicarbonate, which, in turn, is dependent on the duration of hypercapnia. Common etiologies include drug overdose, neuromuscular disease, chest wall abnormalities, and severe airway disorders (eg, asthma, chronic obstructive pulmonary disease [COPD]). Acute hypercapnic respiratory failure develops over minutes to hours; therefore, pH is less than 7.3. Chronic respiratory failure develops over several days or longer, allowing time for renal compensation and an increase in bicarbonate concentration. Therefore, the pH usually is only slightly decreased. The distinction between acute and chronic hypoxemic respiratory failure cannot readily be made on the basis of arterial blood gases. The clinical markers of chronic hypoxemia, such as polycythemia or cor pulmonale, suggest a long-standing disorder (2). Several different abnormalities of breathing function can cause respiratory failure. The major categories, with specific examples of each, are: Obstruction of the airways. Examples are chronic bronchitis with heavy secretions; emphysema; cystic fibrosis; asthma (a condition in which it is very hard to get air in and out through narrowed breathing tubes). Weak breathing. This can be caused by drugs or alcohol, which depress the respiratory center; extreme obesity; or sleep apnea, where patients stop breathing for long periods while sleeping. Muscle weakness. This can be caused by a muscle disease called myasthenia; muscular dystrophy; polio; a stroke that paralyzes the respiratory muscles; injury of the spinal cord; or Lou Gehrig's disease. Lung diseases, including severe pneumonia. Pulmonary edema, or fluid in the lungs, can be the source of respiratory failure. Also, it can often be a result of heart disease; respiratory distress syndrome; pulmonary fibrosis and other scarring diseases of the lung; radiation exposure; burn injury when smoke is inhaled; and widespread lung cancer. An abnormal chest wall (a condition that can be caused by scoliosis or severe injury of the chest wall). Respiratory failure can arise from an abnormality in any of the components of the respiratory system, including the airways, alveoli, CNS, peripheral nervous system, respiratory muscles, and chest wall. Patients who have hypoperfusion secondary to cardiogenic, hypovolemic, or septic shock often present with respiratory failure. Hypoxemic respiratory failure: The pathophysiologic mechanisms that account for the hypoxemia observed in a wide variety of diseases are ventilation-perfusion (V/Q) mismatch and shunt. These 2 mechanisms lead to widening of the alveolar-arterial oxygen difference, which normally is less than 15 mm Hg. With V/Q mismatch, the areas of low ventilation relative to perfusion (low V/Q units) contribute to hypoxemia. An intrapulmonary or intracardiac shunt causes mixed venous (deoxygenated) blood to bypass ventilated alveoli and results in venous admixture. The distinction between V/Q mismatch and shunt can be made by assessing the response to oxygen supplementation or calculating the shunt fraction following inhalation of 100% oxygen. In most patients with hypoxemic respiratory failure, these 2 mechanisms coexist (3). Hypercapnic respiratory failure: At a constant rate of carbon dioxide production, PaCO2 is determined by the level of alveolar ventilation (Va), where VCO2 is ventilation of carbon dioxide and K is a constant value (0.863). (Va = K x VCO2)/PaCO2 A decrease in alveolar ventilation can result from a reduction in overall (minute) ventilation or an increase in the proportion of dead space ventilation. A reduction in minute ventilation is observed primarily in the setting of neuromuscular disorders and CNS depression. In pure hypercapnic respiratory failure, the hypoxemia is easily corrected with oxygen therapy. Hypoventilation, V/Q mismatch, and shunt are the most common pathophysiologic causes of acute respiratory failure. Hypoventilation is an uncommon cause of respiratory failure and usually occurs from depression of the CNS from drugs or neuromuscular diseases affecting respiratory muscles. Hypoventilation is characterized by hypercapnia and hypoxemia. The relationship between PaCO2 and alveolar ventilation is hyperbolic. As ventilation decreases below 4-6 L/min, PaCO2 rises precipitously. Hypoventilation can be differentiated from other causes of hypoxemia by the presence of a normal alveolar-arterial PO2 gradient. V/Q mismatch is the most common cause of hypoxemia. V/Q units may vary from low to high ratios in the presence of a disease process. The low V/Q units contribute to hypoxemia and hypercapnia in contrast to high V/Q units, which waste ventilation but do not affect gas exchange unless quite severe. The low V/Q ratio may occur either from a decrease in ventilation secondary to airway or interstitial lung disease or from overperfusion in the presence of normal ventilation. The overperfusion may occur in case of pulmonary embolism, where the blood is diverted to normally ventilated units from regions of lungs that have blood flow obstruction secondary to embolism. Administration of 100% oxygen eliminates all of the low V/Q units, thus leading to correction of hypoxemia. As hypoxemia increases the minute ventilation by chemoreceptor stimulation, the PaCO2 level generally is not affected. Shunt is defined as the persistence of hypoxemia despite 100% oxygen inhalation. The deoxygenated blood (mixed venous blood) bypasses the ventilated alveoli and mixes with oxygenated blood that has flowed through the ventilated alveoli, consequently leading to a reduction in arterial blood content. The shunt is calculated by the following equation: QS/QT = (CCO2 – CaO2)/CCO2 – CVO2) QS/QT is the shunt fraction, CCO2 (capillary oxygen content) is calculated from ideal alveolar PO2, CaO2 (arterial oxygen content) is derived from PaO2 using the oxygen dissociation curve, and CVO2 (mixed venous oxygen content) can be assumed or measured by drawing mixed venous blood from pulmonary arterial catheter. Anatomical shunt exists in normal lungs because of the bronchial and thebesian circulations, accounting for 2-3% of shunt. A normal right-to-left shunt may occur from atrial septal defect, ventricular septal defect, patent ductus arteriosus, or arteriovenous malformation in the lung. Shunt as a cause of hypoxemia is observed primarily in pneumonia, atelectasis, and severe pulmonary edema of either cardiac or noncardiac origin. Hypercapnia generally does not develop unless the shunt is excessive (>60%). When compared to V/Q mismatch, hypoxemia produced by shunt is difficult to correct by oxygen administration. A majority of patients with respiratory failure are short of breath. Both low oxygen and high carbon dioxide can impair mental functions. Patients may become confused and disoriented and find it impossible to carry out their normal activities or do their work. Marked C02 excess can cause headaches and, in time, a semi-conscious state, or even coma. Low blood oxygen causes the skin to take on a bluish tinge. It also can cause an abnormal heart rhythm (arrhythmia). Physical examination may show a patient who is breathing rapidly, is restless, and has a rapid pulse. Lung disease may cause abnormal sounds heard when listening to the chest with a stethoscope: wheezing in asthma, "crackles" in obstructive lung disease. A patient with ventilatory failure is prone to gasp for breath, and may use the neck muscles to help expand the chest. The symptoms and signs of respiratory failure are not specific. Rather, they depend on what is causing the failure and on the patient's condition before it developed. Good general health and some degree of "reserve" lung function will help see a patient through an episode of respiratory failure. The key diagnostic determination is to measure the amount of oxygen, carbon dioxide, and acid in the blood at regular intervals. A sudden low oxygen level in the lung tissue may cause the arteries of the lungs to narrow. This, in turn, causes the resistance in these vessels to increase, which can be measured using a special catheter. A high blood level of C02 may cause increased pressure in the fluid surrounding the brain and spinal cord. The outlook for patients with respiratory failure depends chiefly on its cause. If the underlying disease can be effectively treated, with the patient's breathing supported in the meantime, the outlook is usually good (4). If the kidneys fail or the diseased lungs become infected, the prognosis is worse. In some cases, the primary disease causing the lungs to fail is irreversible. The patient, family, and physician together then must decide whether to prolong life by ventilator support. Because respiratory failure is not a disease itself, but the end result of many lung disorders, the best prevention is to treat any lung disease promptly and effectively. It is also important to make sure that any patient who has had lung disease is promptly treated for any respiratory infection (even of the upper respiratory tract). Patients with lung problems should also avoid exposure to pollutants, as much as is possible. Once respiratory failure is present, it is best for a patient to receive treatment in an intensive care unit, where specialized personnel and all the needed equipment are available. Close supervision of treatment, especially mechanical ventilation, will help minimize complications that would compound the problem. References 1. Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med 2006; 34(1): 1 – 7. 2. Andrews P, Azoulay E, Antonelli M, e tal. Year in review in intensive care medicine 2005. Acute respiratory failure and acute lung injury, ventilation, hemodynamics, education, renal failure. Intensive Care Med 2006; 32(2): 207 – 16. 3. Broccard AF. Respiratory acidosis and acute respiratory distress syndrome: time to trade in a bull market? Crit Care Med 2006; 34 (1): 229 – 31. 4. Connors AF, Dawson NV, Thomas C, e tal. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med 1996; 154: 959 – 67.