Lab Utilization Worksheet - OPRS Office for the Protection of
advertisement

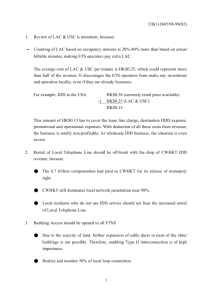
DEPARTMENT OF LABORATORIES AND PATHOLOGY LAC+USC HEALTHCARE NETWORK Laboratory Agreement and Utilization Worksheet Principal Investigator: Email Address: Billing Address: Project Title: IRB Proposal Number: HS - Project Manager: Email Address: Telephone: FAX: Recruitment Locations: Lab Agreement # (to be assigned by Lab): NAME OF TEST NAME OF PERFORMING LABORATORY If this test is not performed by the Clinical Laboratory at LAC+USC Medical Center, please provide address and phone number. - Pager: Number of Number of times per times per patient the test patient the will be done for test will be done for ROUTINE PATIENT RESEARCH CARE TOTAL NUMBER of times per patient the test will be done NUMBER OF PATIENTS Facilities where services will be needed: LAC+USC Medical Center H. Claude Hudson CHC _____ _____ Roybal CHC El Monte CHC _____ _____ With my signature below, I attest this is an accurate listing of the types and number of laboratory tests that will be performed for research and routine patient care during this study. Type or Print Principal Investigator’s Name Signature Date NOTE: 1. Billing will be managed by HSC-CRO (Steve Mackey @ 323-223-4091 x135 for details. 2. LACUSC Department of Pathology will not release the only diagnostic tissue block available and cannot prepare slides for research studies. The USC Translational Pathology Core Facility at USC Norris Cancer Center is available to prepare such slides for a fee, to be shouldered by the individual study. IRB PROPOSAL # Instructions for Completion of Laboratory Agreement and Utilization Worksheet 1. List all clinical laboratory services and tests that will be performed using blood, plasma, serum, urine, body fluids, cultures, cells and tissues. 2. List the name of the laboratory that will be performing each test (LAC+USC Medical Center for tests performed at the LAC+USC Medical Center County Clinical Laboratory). If a test will be performed by another laboratory, list the name, address and phone number. 3. List the number of times per patient that each test will be performed for routine patient care. Routine patient care is testing that would be performed (both type and frequency) if the patient were not participating in this research protocol. 4. List the number of times per patient that the test will be performed for research. Research testing is testing (test type or increased frequency) that would not be performed if the patient was not participating in this research protocol. 5. List the number of patients that will be accrued in the study. PROCEDURE 1. The Laboratory Agreement/Utilization Worksheet request must be signed by the Principal Investigator or designee. 2. Before the Lab Agreement becomes valid, the IRB must have approved the study protocol, including the use of the LAC+USC Clinical Laboratory for the requested testing of and/or services for the study participants in the protocol. Please submit the Laboratory Agreement and Utilization Worksheet form to: Mrs. Lourdes Rodriguez Department of Pathology LAC+USC Medical Center, Clinic Tower, Room A7E Office Number: (323) 409-7154 FAX Number: (323) 441-8193 PRINCIPAL INVESTIGATOR or Designee: Print Signature Date LABORATORY AGREEMENT TO PROVIDE SERVICES Faculty Liaison for Tissue Procurement Date Faculty Liaison for Clinical Laboratory Use Date Other Approval as necessary Date Laboratory Director Date REVISION DATE: 12/7/12