Response to reviewers

Response to reviewers
We would like to thank the reviewers for their insightful comments. Please find below our response.
Reviewer #1 (Comments for the Author (Required)):
This manuscript describes the outcomes of patients treated with Cypher (SES) and
Endeavor (ZES) stents in a large population. Detailed patient characteristics and outcomes were reviewed and analyzed. The authors find a higher rate of target lesion revascularization with ZES without any greater safety than SES.
Although the authors mention the possibility of unmeasured biases by the operators that could confound the results, these possible biases should be explicitly acknowledged.
There is the impression, based on prior human and animal data, that ZES may carry a lower risk of stent thrombosis. The operators might choose the ZES in those patients with intuitive risk factors for stent thrombosis that would not be captured in the adjustment. For instance, there are no measures of medication compliance. A physician may choose ZES if he/she has the sense that the patient might not be compliant, need noncardiac surgery, be at increased risk of bleeding, or have other as yet identified medical problems. Most of the mortality difference is apparent by two months, and stent thrombosis could account for only 1/3 of this difference. This suggests that unmeasured baseline differences become evident early
We have now explicitly acknowledged this in our manuscript, page X: “Still, an important limitation is potential confounding by indication due to lack of randomization of the majority of patients. Based on the assumption of of ZES being a safer choice, an operator might have chosen the ZES in patients with other risk factors for stent thrombosis or death than those obtained in our database. More subtle factors such as the likelihood of non-compliance to medications, a sense of increased risk of bleeding, or the likelihood of future non-cardiac surgery are not obtained in our database.”
Another bias that might influence the choice of ZES over SES is that the Endeavor stent is more deliverable and conformable to the vessel than Cypher. Therefore, the ZES might be used in more difficult anatomies such as tortuous or calcified vessels. These lesions are also more at risk for restenosis and thrombosis, again, leading to worse results with ZES. These more difficult delivery angiographic parameters are not included in adjustment models.
We agree with this reviewer that the ZES, also among the Western Denmark operators, has been considered to be more deliverable and conformable to the vessel than the SES.
Deliverability cannot be assessed in a registry study but was assessed in the SORT OUT
III, where we surprisingly did not find that ZES was more deliverable. Still, the ZES is likely to have been used in more difficult anatomies. We have previously found (Jensen et al. JACC 2007) that procedure time was a better predictor for adverse events than using the description lesion types (A, B1, B2, C). In other words, when procedure time
was included in the statistical model, lesion type was no longer a risk factor. We thus believe that we have included a parameter assessing the complexity of the PCI procedure.
Are renal function and pre-procedural anemia available for these patients?
Creatinine, but not haemoglobin, levels are available and is now stated in table X.
Reviewer #2 (Comments for the Author (Required)):
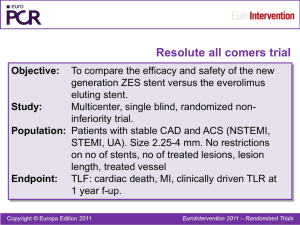
Effectiveness and Safety of Zotarolimus-Eluting and Sirolimus-Eluting Coronary Stents in Western Denmark; Maeng M. ET. Al.
This trial assessed the effectiveness and safety of ZES versus SES in 6181 patients in
Western Denmark followed for 27 months treated between August 2005 and October
2007. 2300 patients received ZES, and 3181 patients received SES. Major adverse cardiac event rates were tabulated and compared. Major findings indicated TLR was greater for ZES compared to SES, (5.3 versus 1.9%). This TLR was due to higher instent restenosis (4.0 versus 1.4%) and stent thrombosis (1.2 versus 0.5%), which were not independently significant. Cardiac mortality and all late safety outcomes were similar including myocardial infarction and stent thrombosis. The authors indicated that
ZES was less effective and not safer than SES. Long-term followup beyond two years was warranted.
This population study focuses attention on differences in TLR in patients receiving ZES versus SES. The large database is a strong factor supporting the conclusions offset by the limitations of such a population based retrospective study in this defined population as well stated by the authors in the first paragraphs of their discussion.
ZES was a proposed as a safer alternative to SES based on the relatively uniform and complete neointimal coverage of the stent struts and reportedly lower incidence of lateacquired incomplete stent apposition thought to protect against late thrombosis. The phosphorylcholine coating used for ZES was thought to be less inflammatory than other polymers used in other stents. With this background as support for the hypothesis, the
Western Denmark Heart Registry (WDHR) was used to examine the outcome of these patients over 27 months of treatment. In patients with ZES, there were 2282 patients treated with 3090 lesions and in patients with SES, there were 3840 patients with 5095 lesions treated. A subset of these patients was randomized into another study called the
Danish Organization for Randomized Trials with clinical outcomes (called Sort Out III) study. Similar medical and specific antiplatelet regimens were provided. Endpoints included target lesion revascularization and target lesion revascularization caused by in-stent restenosis. Safety endpoints included mortality, cardiac mortality, myocardial infarction after 30 days, definitive stent thrombosis and composite of late mortality, late myocardial infarction, and late stent thrombosis. Statistical analysis appears satisfactory.
Specific Comments:
Page 9, Paragraph 1: Results indicate that with regard to the two groups, there was a difference in age with slightly younger patients in the SES group, slightly more male
patients, more smokers, and fewer hypertensives. Please comment on whether these differences are clinically significant to skew the results.
We have now added the following to the manuscript on page X: “………………..”
With regard to the vessels treated, there were more restenotic lesions in the ZES than in the SES group, (2.8 and 1.5%). Since there were more treated in-stent restenotic lesions in ZES, the authors need to consider whether excluding this subgroup changes their event rates. This is a particular subset, which may respond differently to DES than the de novo stenosis. The number of pts receiving more than one stent was also greater in the ZES group. Again, the authors need to indicate whether this was due to the fact that longer lesions were treated or in-stent restenotic lesions required further coverage than non in-stent restenotic lesions.
With regard to hazard ratios of endpoints for cardiac death from Table 3, it appears that there is no significant difference between groups, no significant difference with regard to myocardial infarction, composite endpoint. The TLR was significant, but again for those excluding in-stent restenosis, this value should be provided separately. On a per patient analysis, stent thrombosis was likewise not different, although at the lesion level analysis, stent thrombosis becomes significant (bottom, Table 3). This distinction between patient level and lesion level should be made clear in the text and what clinical significance of this finding is.
If patients with ISR was removed from the analysis, then the estimates of death, ISR, MI, did ………………………………
Page 12, para 2: The issue with regard to in-stent restenosis should be identified.
Usually a discussion of a study emphasizes the findings, the clinical relevance, relationship to previous studies and finally the limitations of the study. The reverse order of the discussion is presented here. Addressing the different interpretations and limitations of such a study at the first opportunity is a novel approach.
Page 12: Paragraph 2 and 3. As above, the discussion of the weaknesses of the study rather than strengths seems awkward. While this is a minor style comment, it does give the pause as to the author's own confidence in their data. The frequent referral to the
Sort Out trial, a pilot of this trial requires some elucidation as to how these 2 data sets differ. Sort Out III findings have been presented only in abstract (reference 25) and are generally not suitable as a reference since it is unpublished data. Likewise for the reference 26, an abstract presented from the Endeavor IV Registry is not generally relied upon without peer review. Caution should be employed when citing this type of data.
The data still support conclusion that ZES are less effective for TLR, but no different with regard to safety endpoints than SES.
Reviewer #3 (Comments for the Author (Required)):
The manuscript from Maeng et al assesses the effectiveness and the safety of zotarolimus-eluting (ZES) and sirolimus-eluting (SES) stent in Western Denmark. The authors included in a multicenter registry a total of 6181 patients (2300 treated with
ZES and 3881 treated with SES) between August 2005 and October 2007 and followed them for up to 27 months. Target lesion revascularization rate was significantly higher
in patients treated with ZES as compared to those receiving SES. Cardiac mortality and late safety outcomes were similar between groups.
Main comments:
- The cohort of patients included in this analysis is a mixture of patients involved in the randomized controlled SORT OUT III trial (30%) and patients treated in the same centers not included in the trial (Registry). Both subgroups of patients may be different in baseline characteristics and outcomes. It would be interesting to know whether this is the case. However, as long as the entire SORT OUT-III trial will be separately analyzed and published, in my view, those patients included in the randomized trial should be excluded from this Registry.
This is an interesting comment as we find that inclusion of the randomized patients strengthens the study’s conclusions . After a second thought, however, the distinction may be relevant and we have subsequently analyzed the outcomes of the nonrandomized patients separately. These results are now reported in the Results section on page X: “……………..”
- In the Results section, table 3, no data regarding myocardial infarction and composite safety endpoint 0-30 days are provided. This is relevant as death and stent thrombosis rates were significantly different only at 30 days and comparable between groups beyond 30 days. Besides, most of the events occurring at early stages after angioplasty are normally attributed to the technique rather than to the type of the stent. On the other hand, target lesion revascularization is the event that could be clearly related to the type of the stent.
In all our previous reports from the Western Denmark Heart Registry we have not reported data on MI within the first month, but have used the definition used by the
MONICA investigators. The explanation is simple and reflects the organization of the
Danish Hospitals. A patient with MI is admitted to Hospital with a Cardiac Care Unit and given the diagnosis MI. The patient is then transferred to one of the invasive hospitals and again given the diagnosis MI. Following PCI the patients are often transferred to another department or back to the referring hospital, and again be given the diagnosis MI. Within the short term, the registry-based diagnosis of MI is thus unreliable as it in most cases will reflect the same MI rather than several MIs.
We agree with the reviewer that events occurring early after PCI are normally attributed to technique rather than type of stent, although we suggest that the rapid release of drug from the phosphorylcholine coating may create a toxic milieu leading to an increased risk of stent thrombosis. This is only a hypothesis and we do not stretch our conclusions with statements saying that SES is safer than ZES, we only conclude that ZES is less effective and not safer.
Minor comments:
Table 2: stent length are almost identical between groups, but the p value appeared
<0.0001. Please check.
Due to the large numbers and relatively little variation, the p value is correct. Similar
“odd” was found in our comparisons of BMS vs DES (REF).
Figures 1A, 1B and 1D: percentage of ZES and SES at 1 month for each event appeared to be 0 in the table below the figure, but in the figure itself the value is higher than 0.
Please check.