Obesity in HSCT Outline 8.17.12 - American Society for Blood and
advertisement

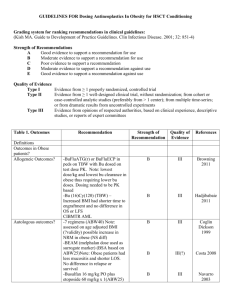
Guidelines for antineoplastic agent dosing in obese patients undergoing HSCT What are the issues to be addressed? Are the outcomes affected in obese patients - Definition of levels of obesity o -% Ideal body weight o - BMI - Outcomes in allos o –Safety (TRM) o –Efficacy (disease control) - Outcomes in autos o –Safety (TRM) o –Efficacy (disease control) How should antineoplastics and immunosuppressant’s be dosed in obese patients - Agents assesssed o Cytotoxics – alemtuzumab, cyclophosphamide, fludarabine, melphalan, clofarabine, etoposide, cytarabine, pentostatin, carmustine, busulfan, thiotepa o Immune modulators – atgam, thymoglobulin, - Actual vs. adjusted body weight o -formula o -% adjustment - Special populations – Elderly, pediatric, Allogeneic- Reduced intensity vs ablative regimens - Ablative - Reduced Intensity - Nonablative Considerations - Different dose limiting toxicities Autologous stem cell transplant o Cytotoxics – Cylophosphamide, melphalan, etoposide, cytarabine, carmustine, busulfan, thiotepa 1 Outcomes References Articles: Barker CC, Agovi MA, Logan B, et al. Childhood Obesity and Outcomes after Bone Marrow Transplantation for Patients with Severe Aplastic Anemia Biol Blood Marrow Transplant 2011;17:737-44 Couglin TM, Kusnierz-glaz CR, Blume K, et al. Impact of admission body weight and chemotherapy dose adjustment on the outcome of autologous bone marrow transplantation. Biol Blood Marrow Transplant 1999;5:299-305 Deeg HJ, Seidel K, Bruemmer B, et al. Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant 1995;15:461-8 Deeg HJ, Seidel K, Sullivan KM Body Weight and Outcome of Hematopoietic Stem Cell Transplantation Am J Med1998;104(6):607-8 Letter Fleming DR, Rayens MK, Garrison J. Impact of obesity on allogeneic stem cell transplant patients: a matched case-controlled study. Am J Med 1997;102:265-8 Fugi S, Kim SW, Yoshimura KI, et al. Possible Association between Obesity and Posttransplantation Complications Including Infectious Disease a d Acute Graft-versusHost Disease Biol Blood Marrow Transplant 2009;15:73-92. Griggs JJ, Mangu PB, Anderson H, et al. Appropriate Chemotherapy Dosing for Obese Adult Patients with Cancer: American Sociery of Clinical Oncology Practice Guideline. J Clin Oncol 2012; 30(13):1553-61. Hadjibabaie M, Tabeefar H, Alimohaddam K, et al. The relationship between body mass index and outcomes in leukemic patients undergoing allogeneic hematopoietic stem cell transplantation Clinical Transplantation 2012;26:149-55. Hansen JA, Gooley TA, Martin PJ, et al Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia N Engl J Med 1998;338:962-8 Navarro WH, Loberiza FR, Bajorunaitte R, et al. Effect of Body Mass Index on Mortality of Patients with Lymphoma Undergoing Autologous Hematopoietic Cell Transplantation Biol Blood marrow Transplant 2006;12:541-51. Navarro WH Impact of obesity in the setting of high dose chemotherapy Bone Marrow Transplant 2003;31:961-6. Navarro WH, Agovi MA, Logan B et al. Obesity Does Not Preclude Safe and Effective Myeloablative Hematopoietic Transplantation (HCT) for Acute Myelogenous Leukemia (AML) in Adults Biol Blood Marrow Transplant 2010;16:1442-50. Nikolousels E, Nagra S, Paneesha S et al. Allogeneic transplant outcomes are not affected by body mass index (BMI) in patients with haematologic malignancies Ann Hematol 2010;89:1141-5 2 Robien K, Schubert MM, Bruemmer B, et al. Predictors of Oral Mucositis in Patients Receiving Hematopoietic Cell transplants for Chronic Myelogenous Leukemia J Clin Oncol 2004;22(7):1268-75. Tarella C, Caracciolo D, Gavarotti P, et al. Overweight as an adverse prognostic factor for non-Hodgkin’s lymphoma patients receiving high-dose chemotherapy and autograft Bone Marrow Transplant 2000;26:1185-91 Wardley AM, Jaysoin GC, Swindell R, et al. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haematopoietic progenitor rescue Br J Haematol 2000;110:292-9 Vogl DT, Wang T, Perez WS, et al. Effect of Obesity on Outcomes after Autologous Hematopoietic Stem Cell Transplantation for Multiple Myeloma. Biol Blood Marrow Transplant 2011;17:1765-74. Overview References Articles: Hanley MJ, Abernathy DR, Greenblatt DJ. Effect of Obesity on the Pharmacokinetics of Drugs in Humans. Clin Pharmacother 2010;49(2):71-87 Langbrake C, Bernhardt F, Baehr M, et al. Drug dosing and monitoring in obese patients undergoing allogeneic stem cell transplantation. Int J Clin Pharm 2011;33:918-24 Sriharsha L, Lipton JH, Pond G, et al. Examining the safety and efficacy of a chemotherapy dosing method in Allogeneic Stem Cell Transplant patients of extreme body size. J Oncol Pharm Practice 2009;15:201-10 Alemtuzumab References Articles: Busulfan References Articles: Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclosphosphamide as a pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000;6:548-54 Andersson BS, Kashyap A, Gian V, et al Conditioning Therapy with Intravenous Busulfan and Cyclophosphamide (IV BuCy2) for Hematologic Malignancies Prior to Allogeneic Stem Cell Transplantation: A Phase II Study. BBMT 2002;8:145-54 Beelen DW, Quabeck K, Graeven U, et al. Acute Toxicity and First Clinical results of intensive postinduction therapy using a modified busulfan and Cyclophosphamide regimen with autologous bone marrow rescue in first remission of acute myeloid leukemia. Blood 1989;74(5):1507-16. 3 Bolinger AM, Zangwill AB, Slatry JL, et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation Bone Marrow transplant 2001;28:1013-8. Booth BP, Rahman A, Dagher R, et al. Population Pharmcokinetic-Based Dosing of Intravenous Busulfan in pediatric Patients J of Clin Pharmacol 2007;47:101-11. Bornhouser M, Storer B, Slattery JT, et al Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic stem cells. Blood 2003;103:820-6 Browning B, Thormann K, Donaldson, A et al. Busulfan Dosing in Children with BMI >85% Undergoing HSCT: A New Optimal Strategy. Biol Blood Marrow Transplant 2011;17:1383-8 Cogle CR, Moreb JS, Leather HL et al. Busulfan, Cyclophosphamide, and Etoposide as Conditioning for Autologous Stem Cell Transplantation in Multiple Myeloma Am J Hematology 2003;73:169-75 Costa LJ, Micalleff IN, Inwardo, DJ, et al. Effect of the dose per body weight of conditioning chemotherapy on severity of mucositis and risk of relapse after autologous haematopoietic stem cell transplantation in relapsed diffuse large B cell lymphoma. Br J Haematol 2008;143:268-37. De Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004;104:857-64 Gibbs JP, Gooley T, Corneau B, et al. The Impact of Obesity and Disease on Busulfan Oral Clearance in Adults Blood 1999;93:4436-40 Grigg A, Harun MH, Szer J. Variability in determination of body weight used for dosing busulphan and cyclophosphamide in adult patients: Results of an international survey. Leuk Lymphoma 1997;25: 487-91. Grochow LB, Krivit W, Whitley CB, et al. Busulfan Disposition in Children Blood 1990;75(8):1723-7 Hadjibabaie M, Tabeefar H, Alimohaddam K, et al. The relationship between body mass index and outcomes in leukemic patients undergoing allogeneic hematopoietic stem cell transplantation Clinical Transplantation 2012;26:149-55. Hoffer E, Akria L, Tabak A, et al. A Simple Approximation for Busulfan Dose Adjustment in Adult Patients Undergoing Bone Marrow Transplantation Ther Drug Monit 2004;26:331-5 Kim JG, Sohn SK, Chase YS et al. Multicenter study of intravenous busulfan, cyclophosphamide, and etoposide (i.v. Bu/Cy/E) as conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin’s Lymnphoma Bone Marrow Transplant 2007;40:919-24 4 Markova M, Barker JN, Miller JS, et al. Fludarabine vs. cladribine plus busulfan and low-dose TBI as reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation: a prospective trial Bone Marrow transplant 2007;39:193-9 McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following Targeted Oral Busulfan as Conditioning for Hematopoietic Cell Transplantation: Pharmacokinetics, Liver Toxicity, and Mortality. Biol Blood Marrow transplant 2007;13:853-62. Meloni G, Proia, A, Capria S, et al. Obesity and autologous stem cell transplantation in acute myeloid leukemia Bone Marrow Transplant 2001;28:365-7 Nguyen L, Leger F, Lemon S, et al. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation a population based study. Cancer Chemother Pharmacol 2006;57:191-8 Russel JA, Tran HT, Quinlan D, et al. Once daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early outcomes. Biol Blood Marrow Transplant 2002;8:468-76 Schiltmeyer B, Klingebiel T, Schwab M, et al Population pharmacokinetics of oral busulfan in children Cancer Chemother Pharmacol 2003;52:209-16 Shaughnessy P, Alexander W, Tran H, et al. Phase I and Pharmacokinetic Study of Once-Daily Dosing of Intravenously Administered Busulfan in the Setting of RedecedIntensity Preparative Regimen and Allogeneic Hematopoietic Stem Cell Transplantation as Immunotherapy for Renal Cell Carcinoma Military Medicine 2006:171(2):161-5 Sucak GT, Suyani E, Baysal NA, et al. The role of body mass index and other body composition parameters in early post-transplant complications in patients undergoing allogeneic stem cell transplantation with busulfan-cyclophosphamide conditioning Int J Hematol 2012;95:95-101 Takama H, Tanaka H, Nakashima D, et al. Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation Bone Marrow Transplant 2006;37:345-51 Thomas ED, Sanders JE, Flournoy N et al Marrow Transplantation for patients with acute lymphoblastic leukemia in remission. Blood 1979;54:468-76. Trame M, Bergsstrand M, Mats O, et al Population Pharmacokinetics of Busulfan in Children: Increased Evidence for Body Surface Area and Allometric Body Weight Dosing of Busulfan in Children Clin Cancer Res 2011;17:6867-6877 Tutschka, PJ, Copelan EA, Klein JP. Bone marrow Transplantation for Leukemia Following a New Busulfan and Cyclophosphamide Regimen Blood 1987;70(5):1382-8 Carboplatin References 5 Carmustine References Articles: Costa LJ, Micalleff IN, Inwardo, DJ, et al. Effect of the dose per body weight of conditioning chemotherapy on severity of mucositis and risk of relapse after autologous haematopoietic stem cell transplantation in relapsed diffuse large B cell lymphoma. Br J Haematol 2008;143:268-37. Clofarabine References Articles: Cyclophosphamide References Articles: Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclosphosphamide as a pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000;6:548-54 Andersson BS, Kashyap A, Gian V, et al Conditioning Therapy with Intravenous Busulfan and Cyclophosphamide (IV BuCy2) for Hematologic Malignancies Prior to Allogeneic Stem Cell Transplantation: A Phase II Study. BBMT 2002;8:145-54 Beelen DW, Quabeck K, Graeven U, et al. Acute Toxicity and First Clinical results of intensive postinduction therapy using a modified busulfan and Cyclophosphamide regimen with autologous bone marrow rescue in first remission of acute myeloid leukemia. Blood 1989;74(5):1507-16. Grigg A, Harun MH, Szer J. Variability in determination of body weight used for dosing busulphan and cyclophosphamide in adult patients: Results of an international survey. Leuk Lymphoma 1997;25:487-91. Hadjibabaie M, Tabeefar H, Alimohaddam K, et al. The relationship between body mass index and outcomes in leukemic patients undergoing allogeneic hematopoietic stem cell transplantation Clinical Transplantation 2012;26:149-55. Meloni G, Proia, A, Capria S, et al. Obesity and autologous stem cell transplantation in acute myeloid leukemia Bone Marrow Transplant 2001;28:365-7 Tutschka, PJ, Copelan EA, Klein JP. Bone marrow Transplantation for Leukemia Following a New Busulfan and Cyclophosphamide Regimen Blood 1987;70(5):1382-8 Thomas ED, Sanders JE, Flournoy N et al Marrow Transplantation for patients with acute lymphoblastic leukemia in remission. Blood 1979;54:468-76. Cytarabine References Articles: 6 Costa LJ, Micalleff IN, Inwardo, DJ, et al. Effect of the dose per body weight of conditioning chemotherapy on severity of mucositis and risk of relapse after autologous haematopoietic stem cell transplantation in relapsed diffuse large B cell lymphoma. Br J Haematol 2008;143:268-37. Etoposide References Articles: Costa LJ, Micalleff IN, Inwardo, DJ, et al. Effect of the dose per body weight of conditioning chemotherapy on severity of mucositis and risk of relapse after autologous haematopoietic stem cell transplantation in relapsed diffuse large B cell lymphoma. Br J Haematol 2008;143:268-37. Wolff SN, Johnson DH, Hainsworth JD, et al. High-dose VP-16-213 Monotherapy for refractory germinal malignancies: A phase II study. J Clin Oncol 1984;2(4):271-4. Fludarabine References Articles: Quinlan D, et al. Once daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early outcomes. Biol Blood Marrow Transplant 2002;8:468-76 Melphalan References Articles: Costa LJ, Micalleff IN, Inwardo, DJ, et al. Effect of the dose per body weight of conditioning chemotherapy on severity of mucositis and risk of relapse after autologous haematopoietic stem cell transplantation in relapsed diffuse large B cell lymphoma. Br J Haematol 2008;143:268-37. Grazziutti ML, Dong L, Micelli MH et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: Incidence, risk factors, and a severity predictive model. Bone Marrow Transplant 2006;38:501-6 Pentostatin References Articles: Thiotepa References Articles: Atgam References Articles: Thymoglobulin References 7 Articles: 8