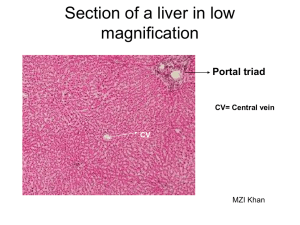
23.Surgical diseases of the liver
advertisement

THE KURSK STATE MEDICAL UNIVERSITY DEPARTMENT OF SURGICAL DISEASES № 1 SURGICAL DISEASES OF THE LIVER Information for self-training of English-speaking students The chair of surgical diseases N 1 (Chair-head - prof. S.V.Ivanov) BY ASS. PROFESSOR I.S. IVANOV KURSK-2010 DEVELOPMENT OF HEPATOBILIARY SURGERY Surgeons have learned to operate successfully on the liver primarily during the past three decades. For centuries, the liver was a mysterious organ with complex anatomy, an overwhelming number of functions, and an extraordinary capability to regenerate. The organ's large size and abundant blood supply contributed to the respect paid to this organ in most civilizations and operating theaters. Improved understanding of anatomy and physiology, combined with a number of recently developed surgical techniques, led from myth and mystery to the emergence of the specialty of hepatobiliary surgery. Laparoscopic cholecystectomy rivals Langenbuch's contribution of the open technique (he performed the first successful cholecystectomy in 1882) with respect to surgical importance. Not only has laparoscopic cholecystectomy opened the field to other new procedures, laparoscopic surgery has contributed greatly to present interest in shortened hospital stays, lessened costs, and the rethinking of surgical dogma such as wide exposure. The development of hepatobiliary surgery culminates in the rise and increased safety of hepatic resections and liver transplantation. A large number of hepatic resections are performed by specialized surgeons in major centers. The mortality of elective resection has decreased from 20% two decades ago to less than 1%. This increased safety follows improved technology and understanding of the anatomy and physiology of the liver. With improved safety has come an increased confidence in liver surgery, a wide expansion of the indications for resection, and development of other aggressive procedures such as cryoablation and chemoembolization. The most common indication for partial liver resection in most centers remains neoplasia. A spectacular advance in hepatic surgery and hepatic therapy in general has been the success of liver transplantation. Welch performed the first experimental liver transplant in 1955 using a heterotopic technique in dogs, but the procedure was abandoned because of difficulties in maintaining vascular inflow and adequate biliary drainage. In 1959, Moore and Starzl independently achieved successful orthotopic liver transplantation in dogs, the same year that Kasai and Suzuki reported the first hepatoportoenterostomy for biliary atresia. The first human liver transplant was performed by Starzl in 1963. Subsequently, Starzl and Calne developed large series of liver transplants. Other important advances in transplantation include identification of the immunosuppression characteristics of cyclosporine by Borel in 1972 and the clinical trials of newer agents such as tacrolimus (FK506) in the 1990s. An important achievement, which combined the advances in hepatic resection and transplantation, was the successful transplantation of the left lateral segment from a live parent to a child, performed in 1989 at the University of Chicago by Broelsch and Emond. Success with monitoring human life using a pig liver suggests there is great promise for xenotransplantation, perhaps with transgenic livers. It is interesting to note the exchange of roles surgery and medicine have had in the treatment of hepatobiliary disorders over the past two decades. The development of endoscopic and endosurgical approaches has radically changed the treatment of gallstones. Many of the traditional medical disorders such as cirrhosis and metabolic deficiencies are being treated by liver transplantation. The development of minimally invasive techniques for hepatobiliary diseases raises important new questions with respect to the definition of surgery. It is likely the next decade will see consolidated educational programs for the training of physicians and surgeons in this field. A unified International Hepato-PancreatoBiliary Association has led to the development of the American Hepato-Pancreato-Biliary Association (AHPBA), which promotes a union of surgeons, gastroenterologists, radiologists, and other specialists who work within this dynamic field. I. ANATOMY AND PHYSIOLOGY ANATOMY AND PHYSIOLOGY Modern concepts of gross hepatobiliary anatomy differ considerably from the anatomy suggested by the ligamentous reflections of the peritoneum, particularly the falciform ligament. For centuries the right lobe of the liver was defined as all the hepatic parenchyma to the right of the falciform ligament and the left lobe as only the substance to the left of the ligament. There are now two new classifications of the gross anatomy that have much more applicability to surgery. The first is the lobar system used most frequently in the United States and often called the American System. The second is the French segmental system, which has the most applicability. Anatomic features that enable the liver to be an important integrator between the digestive system and the rest of the body include (1) a dual blood supply, with portal blood from the splanchnic system and the hepatic artery; (2) a specific architectural arrangement of single cells and cell masses that facilitates exchange between blood and hepatocytes; (3) a specific orientation of the hepatocytes that compartmentalizes biliary versus blood pathways; and (4) an organized biliary excretory system that regulates the enterohepatic circulation. In this section aspects of the anatomic organization of the liver are considered that are important for both hepatic physiology and surgery. Gross Anatomy General Description The liver lies in the right upper quadrant of the abdomen, beneath the diaphragm and connected to the digestive tract by means of the portal vein and the biliary drainage system. The largest gland in the body, it weighs approximately 1500 gm. in the adult . The liver accounts for 2% of the body weight of the adult and about 5% of the body weight of a newborn. Hepatic extramedullary hematopoiesis produces the relatively larger liver size in newborns. The normal adult liver resides under the protective rib cage. It extends in the midclavicular line from as high as the fourth intercostal space down to slightly below the costal margin. The gallbladder lies on the dorsal surface of the liver in a transpyloric plane. A peritoneal membrane (Glisson's capsule) covers the liver and extends as fibrous septa into the parenchyma with blood vessels and bile ducts. The superior surface of the liver conforms to the undersurface of the right diaphragm. Only the liver to the left of the falciform ligament contacts the left diaphragm. The inferior surface of the liver touches the duodenum, colon, kidney, adrenal gland, esophagus, and stomach. Peritoneum invests the entire liver except for a bare area under the diaphragm on the posterosuperior surface adjacent to the inferior vena cava and hepatic vein. Normal Development The liver primordium appears at about the third week as a ventral thickening of the entoderm at the distal end of the foregut (future duodenum). The major portion of this primordium produces hepatic parenchyma and the main bile duct. A secondary caudal proliferation will become the gallbladder and cystic duct. The hepatic primordium is formed of cellular cords, which colonize the ventral mesogastrium (septum transversum). The vitelline (omphalomesenteric) veins connected to the digestive tube consist of an anastomotic network around the duodenum and then cross the septum transversum. Proliferation of the entodermal cords forming the hepatic primordium fragments the vitelline veins into a vascular labyrinth: the hepatic sinusoids. The hepatocytes arrange themselves into cords surrounding the sinusoidal capillaries. When the yolk sac disappears, the vitelline veins regress almost totally and persist only in their mesenteric branches. Caudad to the liver, the anastomotic network of vitelline veins fuse into a single trunk, the portal vein. In a cranial direction the vitelline veins open into the sinus venosus. When the left horn of the sinus venosus disappears, the right vitelline trunk receives the anastomosis of the inferior vena cava and becomes the terminal segment. Extension and proliferation of hepatocytes into the entire septum transversum result in concurrent fragmentation of the umbilicoallantoic veins (more lateral to the vitelline veins). The right umbilicoallantoic vein regresses in the sixth week, leaving the left one to drain blood coming from the placenta to the liver. The left umbilical vein drains into the left portal vein and passes through a temporary, short circuit (ductus venosus) directly into the inferior vena cava. The ductus venosus and left umbilical vein are obliterated after birth to form the ligamentum venosus and the ligamentum teres. The hepatocytes proliferate, and the liver protrudes from the transverse septum into the abdomen, with the bare area a reminder of its origin. Bile ducts differentiate from hepatic cells and join the extrahepatic biliary system, appearing first in the hilum and then spreading peripherally. Bile formation may be evident as early as the third month. Topographic Anatomy The reflections of peritoneum that attach the liver to the abdominal wall, diaphragm, and abdominal viscera determine the topographic anatomy of the liver. Three sets of ligaments include the following: 1. The falciform ligament, which attaches the liver to the anterior abdominal wall from the diaphragm to umbilicus and incorporates the ligamentum teres hepaticus in its dorsal border. In persons with portal hypertension, the umbilical vein recanalizes and connects the periumbilical superficial venous system with the portal system. 2. The anterior and posterior right and left coronary ligaments, which in continuity with the falciform ligament connect the diaphragm to the liver. The lateral aspects of the anterior and posterior leaves of the coronary ligaments fuse to form the right and left triangular ligaments. The area encompassed by the falciform, coronary, and triangular ligaments over the inferior vena cava and under the diaphragm is the bare area of the liver. 3. The gastrohepatic and hepatoduodenal ligaments, which consist of the anterior layer of lesser omentum and are continuous with the left triangular ligament. The hepatoduodenal ligament contains the hepatic arteries, portal vein, and extrahepatic bile ducts. It forms the anterior boundary of the epiploic foramen of Winslow and the communication between the greater and lesser peritoneal cavities. Four lobes of the liver are commonly described: right, left, quadrate, and caudate. The topographic right lobe includes a portion of the liver to the right of the falciform ligament and the topographic left lobe portion to the left. The quadrate lobe is a rectangular junction on the inferior surface bounded by the umbilical fissure on the left, the gallbladder fossa on the right, and the portal triad posteriorly. The posterior (transverse) extension of the falciform ligament (ligamentum venosum) on the left and the impression of the inferior vena cava on the right delineate the caudate (spigelian) lobe. Lobar Anatomy (The American System) The distribution of the major branches of the veins, arteries, or bile ducts of the liver does not conform precisely with the topographic anatomy . The general relationships between the hepatic veins and portal vein branches determine the lobar anatomy of the liver, which is best demonstrated by direct injection of its blood supply with substances such as methylene blue or colored celloidin. A plane called the portal fissure (Cantlie's line) passes from the left side of the gallbladder fossa to the left side of the inferior vena cava to divide the liver into right and left lobes. The left lobe consists of a medial segment, which lies to the right of the falciform ligament and umbilical fissure, and a lateral segment, which lies to the left of the falciform ligament. The right lobe consists of an anterior and a posterior segment. No visible surface marking delineates the lobar segmental anatomy. Conventionally, most of the topographic caudate lobe is in the medial segment of the left lobe, but it extends over the plane between the gallbladder and the inferior vena cava into the anatomic right lobe. The conceptual division of the liver into lobes and segments forms the basis for the four classic types of major hepatic resection . The lobes may be further divided into subsegments that correspond to segments in the French system, described next. French Segmental System Another nomenclatural system for hepatic anatomy was developed by Soupault and Couinaud. This system shows more consideration for the hepatic venous drainage and caudate lobe but also applies to the portal, biliary, and arterial anatomy. Instead of four, there are eight segments: four on the right, three on the left, and one corresponding to the topographic caudate lobe. Segment I corresponds to the caudate lobe; segments II to IV constitute the left lobe; and segments V to VIII the right lobe. The three main hepatic veins divide the liver into four sectors. The planes containing the right, middle, and left hepatic veins are called portal scissurae, while the planes containing portal pedicles are called hepatic scissurae. The caudate lobe is its own autonomous segment in the French system. In general, the segments described in the French classification correspond to the subsegments described in the lobar anatomic classification. Portal Vein The portal vein provides about three fourths of the liver's blood supply. The junction of the superior mesenteric and splenic veins forms the portal vein, dorsal to the neck of the pancreas. The portal vein then passes superiorly, posterior to the first part of the duodenum at the level of the second lumbar vertebra. This vein varies from 1 to 3 cm. in diameter and 5 to 8 cm. in length before dividing into right and left branches at the porta hepatis. In about 10% of persons there appear to be three main trunks of the portal vein, with two going to the right lobe and one to the left lobe. The extra trunk represents the right branch of the portal vein dividing into segmental (sectoral) branches before entering the liver. The portal vein usually passes behind the bile duct and hepatic artery in the hepatoduodenal ligament. The portal vein rarely varies. Although the portal vein neatly divides into right and left branches, it does not distribute splanchnic blood equally to the hepatic lobes. Radioactive phosphorus injected into the superior mesenteric vein preferentially flows into the right lobe, whereas splenoportography demonstrates the two lobes nonselectively. Despite the preferential flow, the two lobes function similarly. However, the preferential flow has pathologic significance; for example, amebic abscesses appear predominantly in the right lobe. The portal trunk divides into left and right hepatic branches in the portal fissure. The left branch of the portal vein is longer and consists of two portions: (1) the pars transversus, which traverses the base of segment IV, and (2) the pars umbilicus, which turns into the umbilical fissure. Two branches to the lateral segment of the left lobe (segments II and III) usually arise from the pars umbilicus near the plane of the falciform ligament. Branches from both the pars transversus and umbilicus supply the medial segment of the left lobe (segment IV). The right branch of the portal vein divides into anterior and posterior segments approximately at the point of entry into liver parenchyma. The portal vein divides into small veins and venules, which finally enter hepatic sinusoids. Abundant vascular intercommunications exist at the sinusoidal level. The absence of portal vein valves has several important implications: (1) pressures observed in portal vein tributaries reflect portal vein pressure, and therefore, during surgery for portal hypertension, portal pressure is conveniently measured in a small mesenteric or omental vein; (2) the intrahepatic portal vein's low resistance sustains a large amount of flow despite loss of much kinetic energy to the capillary network of the digestive system; and (3) the specialized intrahepatic architecture accommodates both the high-pressure hepatic arteries and the portal veins. Numerous tributaries of the portal vein connect outside the liver with the systemic venous system. Under normal circumstances these communications have little physiologic significance. However, if portal hypertension develops, these rudimentary portosystemic communications develop into large channels with increased collateral flow. The most important natural portosystemic anastomoses include (1) the submucosal veins of the proximal stomach and distal esophagus, which can receive blood from the coronary and short gastric veins to drain into the azygous veins (high blood flow through this pathway produces gastric varices, esophageal varices, or both); (2) umbilical and periumbilical veins, recanalized from the obliterated umbilical vein in the ligamentum teres hepaticus, and which may cause spectacular physical findings such as caput medusae or the loud Cruveilhier-Baumgarten bruit; (3) tributaries of the inferior mesenteric vein, which include the superior hemorrhoidal veins that communicate with the middle and inferior hemorrhoidal veins of the systemic circulation and may cause large hemorrhoids; and (4) other retroperitoneal communications, including connections to the renal and adrenal veins. Hepatic Artery The extrahepatic arterial system does not parallel the portal channels, although the intrahepatic system does. Over 50% of the population have the same anatomic pattern. The hepatic artery arises from the celiac axis and passes along the upper part of the pancreas toward the liver. Posterior and superior to the duodenum it gives off the gastroduodenal artery. The terms proper or common hepatic arteries refer to the segment proximal or distal to the origin of the gastroduodenal artery. Note that some anatomic textbooks denote those terms in reverse (proper: distal; common: proximal to the gastroduodenal artery). Therefore, using the entire phrase “hepatic artery proximal or distal to the gastroduodenal artery” is suggested. Within the hepatoduodenal ligament the hepatic artery divides into right and left branches and subsequently into smaller branches corresponding to the portal venous system, segmental, or subsegmental anatomy. Because of abundant collaterals, ligation of the hepatic artery proximal to the gastroduodenal artery fails to damage the liver. Ligation of the hepatic artery distal to the gastroduodenal artery can produce hepatic necrosis and death but also may not result in serious consequences because of development of a rich collateral extrinsic blood supply from the celiac axis, superior mesenteric, and inferior phrenic arteries. Ligation of the right or left hepatic artery usually results in elevated enzyme levels but often without severe clinical manifestations. A diffuse subcapsular arterial plexus may contribute significantly to the hepatic arterial collateral circulation. One angiographic study has shown that rich collaterals can also develop in the liver's suspensory ligaments. The most important variations of the hepatic arterial system are a right hepatic artery and a common hepatic artery arising from a superior mesenteric trunk (replaced hepatic arteries). Other anomalies include the left hepatic artery arising from the left gastric artery, the right hepatic artery traveling anterior rather than posterior to the bile duct, and the right hepatic artery traveling posterior to the portal vein. In addition, the right hepatic artery often has a curved extrahepatic course, which may lead to inadvertent ligation during cholecystectomy. The cystic artery usually arises from the right hepatic artery but occasionally arises from the gastroduodenal artery, the left hepatic artery, or the common hepatic artery. Double cystic arteries occasionally occur. When significant hepatic arterial branches arise from the superior mesenteric artery, they usually pass to the right side of and posterior to the portal vein. Hepatic Veins Most of the hepatic venous effluent drains into the three major hepatic veins—right, middle, and left. Each has only a short extrahepatic segment before draining into the inferior vena cava. In general, the short extrahepatic segment makes surgical accessibility difficult, particularly for control of traumatic bleeding. The right hepatic vein, the largest of the three, provides the principal drainage for the right lobe of the liver. The main trunk of the right hepatic vein follows an intersegmental plane between the French segments or the anterior and posterior segments (American system). Several small veins also normally drain directly from the right lobe into the vena cava. The middle hepatic vein lies in the lobar (portal) fissure draining the medial segment of the left lobe and a portion of the anterior segment of the right lobe. The middle hepatic vein joins the left hepatic vein in 80% of dissections. The exact site of juncture varies considerably. The left hepatic vein provides the principal venous drainage of the left lateral segment. In addition, several small veins from the caudate lobe drain posteriorly directly into the vena cava. After thrombosis of the major hepatic veins (Budd-Chiari syndrome), these small posterior caudate veins become particularly important. Biliary System The biliary drainage system begins at the hepatocyte level, where portions of the hepatocyte membrane form small channels called canaliculi. Bile drains from the canaliculi into intrahepatic ducts that follow the segmental anatomy determined primarily by the vascular supply. The convergence of canaliculi and proximal ductal systems is called the canal of Hering. The ductal pattern becomes more variable distally. The left lobar duct forms in the umbilical fissure from the union of ducts from segments II, III, and IV, then passes to the right across the base of segment IV (medial segment of the left lobe, topographic quadrate lobe), and unites with the right lobar duct to form the common hepatic duct. The right hepatic duct drains segments V and VIII and arises usually from the junction of the anterior and posterior segmental (sectoral) ducts. The right posterior duct usually follows almost a horizontal course before joining with the anterior duct, where it descends more vertically. The junction of the two main right biliary channels is usually found above the right branch of the portal vein. The shorter extrahepatic right lobar duct joins the longer left duct at the base of the right lobe. The extrahepatic portion of the left lobar duct characteristically is about 2 cm. long. The right and left lobar ducts join outside the liver to become the common hepatic duct, which passes anterior to the portal vein in most persons. The left hepatic duct joins the right hepatic duct at a much more anterior and acute angle, which is an anatomic consistency of importance during exploration of the common duct or cholangiography. The length of the common hepatic duct varies according to the location of its junction with the cystic duct, where it becomes the common bile duct. The hepatic duct confluence varies considerably with respect to the union of the right and left main hepatic ducts. The biliary drainage of the topographic caudate lobe (segment I) varies considerably but enters both the right and left hepatic duct systems in about 80% of persons. In about 15% of cases, the caudate lobe drains only into the left hepatic duct system, and in about 5% it drains only into the right hepatic duct. The upper limit of normal for the diameter of the common bile duct is controversial. Most references give the upper limit as 6 to 8 mm. except after cholecystectomy, when the common bile duct may dilate to 10 to 12 mm. Intrahepatic and extrahepatic ducts usually lie anterior to the corresponding portal branches. The extrahepatic bile ducts lie within the hepatoduodenal ligament. The common hepatic artery ascending to the left of the common bile duct gives off the right hepatic artery, which usually courses dorsal to the bile duct. Like the common hepatic duct, the common bile duct varies in length. It passes posterior to the first part of the duodenum and then courses through the pancreas and the wall of the duodenum to form the papilla of Vater on the medial duodenal wall. The major pancreatic duct (duct of Wirsung) joins the common duct in about 90% of cases, forming the ampulla of Vater. Sphincter of Oddi. The circular smooth muscle fibers in the ampulla of Vater area constitute the sphincter of Oddi, which regulates the flow of bile from the liver into the duodenum. The three principal parts of the sphincter of Oddi are the sphincter of the choledochus (i.e., the circular muscle fibers surrounding the intramural and submucosal bile duct); the pancreatic sphincter, which consists of the amuscular septum between the bile and pancreatic ducts; and an ampullary sphincter. The ampullary sphincter, the most important component of the sphincter of Oddi, includes a layer of longitudinal muscle fibers that help prevent reflux of intestinal contents into the ampulla. Relaxation of the ampullary sphincter may promote reflux into the pancreatic duct. About 10% of papillas have clearly distinct, separate openings of the common bile duct and pancreatic duct. The blood supply of the common bile duct arises from the gastroduodenal, common hepatic, and right hepatic arteries. A plexus formed on the duct provides two axial vessels, the 3 o'clock and 9 o'clock arteries, named for their positions relative to a cross-section of the duct. Gallbladder. The gallbladder, a pear-shaped, distensible appendage of the extrahepatic biliary system, usually holds 30 to 50 ml. of bile. It has a fundus, body, and neck. The gallbladder fills and empties through the cystic duct, which varies in length and usually contains spiral valves of Heister that regulate bile flow. The valves may be extremely tortuous, complicating cannulation during intraoperative cholangiography. Enlargement of the neck of the gallbladder such as from a stone may form a pouch (Hartmann's pouch). The triangle bounded by the cystic duct, common hepatic duct, and inferior border of the liver is the triangle of Calot. The gallbladder receives its blood supply from the cystic artery, which originates from the right hepatic artery, usually after the latter passes beneath the common hepatic duct. Venous drainage of the gallbladder enters principally into the portal vein. The lymphatics drain into cystic duct nodes near the superior aspect of the cystic duct. Venous and lymphatic channels also enter into the liver parenchyma. Biliary System Variants. Variations in the gallbladder and related anatomy are important during surgery, because failure to recognize variants can produce iatrogenic injury . Small accessory ducts (ducts of Luschka) between the liver and gallbladder easily escape detection. Low extrahepatic right segmental duct insertions can also join the cystic duct. The latter injury has been recognized since the use of laparoscopic cholecystectomy. Occasionally, liver parenchyma is partially embedded in the gallbladder, and rarely one may encounter a completely intrahepatic gallbladder. The length of the cystic duct varies, and it occasionally passes for several centimeters ensheathed with the common hepatic duct. Passage of the cystic duct posterior and around the common hepatic duct to form a left-sided junction (spiral union) occurs in less than 5% of persons. The cystic duct may also join the right or left hepatic duct or be absent. Rarely, major hepatic ducts drain separately into the gallbladder. Common variations in the anatomy of the hepatic artery of relevance to this biliary anatomy include a bend in the course of the hepatic artery, which can mimic the cystic artery origin, a short cystic artery takeoff from the right hepatic artery, dual cystic arteries, or an artery that courses anterior to the hepatic ductal system. Nerves The portal and pericapsular regions harbor a distinct, complex system of nerves of unknown clinical importance. An anterior neural plexus consists primarily of sympathetic fibers derived bilaterally from ganglia T7 to T10 and synapsing in the celiac plexus or of fibers from the right and left vagus and right phrenic nerves. The anterior plexus surrounds the hepatic arteries. A posterior plexus that intercommunicates with the anterior plexus lies around the portal vein and bile ducts. The sympathetic nerves innervate the hepatic arteries. Distention of the liver capsule or gallbladder causes pain referred to the right shoulder or scapula by means of the third and fourth cervical nerves. Interruption of the anterior neural plexus may have various physiologic effects, such as on the composition of secreted bile and the accumulation of fat in the liver. The significance of these findings is not known. Lymphatics Hepatic lymph forms in the perisinusoidal spaces of Disse and in the clefts of Mall to drain into larger lymphatics in the porta hepatis, subsequently into the cisterna chyli, and eventually into the thoracic duct. Lymphatic vessels also lie near the hepatic vein in Glisson's capsule and around the bile ducts. Lymphatics also pass through the diaphragm into the thoracic duct. Hepatic lymph nodes are found in the porta hepatis, celiac region, and near the inferior vena cava. Cirrhosis, veno-occlusive disease, and glycogenosis produce lymph vessel dilation. Alterations in the permeability of sinusoidal epithelial cells can alter lymph flow and protein content, an observation important in the pathogenesis of ascites. Injection of dye into the bile duct under supraphysiologic pressure reveals communication with the hepatic lymph vessels, but the importance of these communications under physiologic conditions is unknown. Anomalous Development of the Liver Incomplete or maldevelopment of the hepatobiliary system can cause a number of anomalies that are encountered clinically. Complete absence of the liver is rare and not reported after birth. Absence of the left lobe has been seen. Hepatic transposition has been reported with situs inversus. Occasionally, a tongue of liver tissue extends inferiorly from the right lobe (Riedel's lobe); more frequently encountered in the female, this condition usually causes no symptoms, although it may be associated with colonic or pyloric obstruction. Heterotopic liver tissue has been seen in the gallbladder, pancreas, adrenals, spleen, or within an omphalocele. Four cases of supradiaphragmatic liver have been reported in the absence of a hernia sac. Although biliary variants are extremely common, true anomalies are not. Biliary atresia and choledochocele are the most common serious biliary problems seen after birth. Other abnormalities include congenital absence of gallbladder, intrahepatic or left-sided gallbladder, multiple gallbladders, and abnormalities in the shape of the gallbladder. The definition of multiple gallbladders is determined by the presence of more than one cystic duct. Other gallbladder anomalies include septation, bilobation, and duplication of the cystic duct. Occasionally the gallbladder has a long mesentery predisposing to torsion. Portal vascular abnormalities include portal agenesis, congenital portocaval shunt, a preduodenal portal vein, and anomalous pulmonary veins that traverse the diaphragm and enter the portal system. Microscopic Anatomy The Acinar Unit The smallest functional unit of the liver is the acinus, a structure first named by Rappaport . In an acinar unit, the portal venule is accompanied by a hepatic arteriole, a bile ductule, lymphatics, and nerves. Blood flows from the terminal portal venules into the sinusoids and comes in contact with hepatocytes in the unit. The blood drains into the terminal hepatic venule. The solutes are removed by the hepatocytes, and their concentration decreases as the blood flows toward the terminal hepatic venule. The hepatocytes around the portal venule axis are divided arbitrarily into three zones. In zone 1, the area immediately adjacent to the portal venule, the sinusoids are smaller in diameter and more anastomotic than in zones 2 and 3, which are farther away from the portal venule. This concept explains centrilobular necrosis that occurs with hypotension; zone 1 cells are the first to receive blood and oxygen and the last to experience hypoxia. Microcirculation The terminal branches of the major inflow system (the portal vein) and the major outflow system (the hepatic vein) do not meet but are regularly interspersed with space between them filled with hepatic cell plates and sinusoids. The portal veins and their branches become progressively smaller as they penetrate the liver substance, while the terminal hepatic veins connect with the sinusoidal bed, piercing through closely applied cell plates. This anatomy is clearly a result of the embryologic development of the liver. Flow of portal blood in the sinusoids is partially regulated via the periphery of the cell plates. The branches of the hepatic artery similarly decrease in caliber as they penetrate the parenchyma, and their size and composition change accordingly. In the terminal branches, they form a general plexus, which eventually terminates in the sinusoids. A special capillary plexus encompasses the bile ducts; this network also terminates in the sinusoids. The peribiliary plexus appears to play an important role in ductular bile secretion and absorption. Cuffs of smooth muscle surrounding the terminal arterioles help regulate the flow into the sinusoids . The hepatic sinusoids are 7 to 15 mm. wide, but the width may increase to 180 mm. under physiologic conditions. Pressure within the sinusoids is only 2 to 3 mm. Hg, making this an extremely low resistance system. Three anatomic features characterize this low resistance system: 1. The sinusoids are lined by Kupffer's and endothelial cells, which overlap loosely and are not attached to one another. The stellate Kupffer's cell cannot be distinguished from endothelial cells by light microscopy. However, the former are much larger, and have irregular surfaces with folds and microvilli. 2. The endothelial cells are flat and fenestrated, with openings varying from 0.1 to 2 mm. in diameter. 3. Hepatocyte and membrane microvilli project through the fenestrations of the endothelial cells and therefore facilitate and maximize hepatocyte exposure to sinusoidal contents. The space between the endothelial lining of the sinusoid and the hepatocyte is the perisinusoidal space of Disse. Important cells located in the perisinusoidal space of Disse include fibroblasts, Ito cells (lipocytes), and neurons. Sinusoids are freely permeable to low- and high-molecular-weight substances in solution. The sinusoids empty into terminal hepatic venules, which in turn empty into hepatic veins of increasing caliber. The space of Disse is the primary site for the formation of hepatic lymph. Hepatocytes and Sites of Bile Formation Hepatocytes represent approximately 60% of the cells of the adult human liver and 80% of the cytoplasmic mass. These diameters vary between 13 and 30 mm. The cells have both a microvillar and smooth surface, with the microvillar surface lining the perisinusoidal space. The smooth surface is capable of forming microvilli, particularly during regeneration and cholestasis. The microvilli of the hepatocytes are not as extensive as the microvilli of the intestinal epithelial lining, increasing the surface area 1.6-fold compared with 24-fold for the latter. Approximately 15% of the plasma membrane encompasses bile canaliculi that remain separated from the pericellular space by tight junctions and desmosomes. The main source of canalicular bile is across the canalicular membrane, although some paracellular flow of small solutes also occurs. The canaliculi measure approximately 1 mm. in diameter. The epithelial ductular or ductular lining cells measure 10 mm. in diameter and have a distinct basement membrane, in contrast to hepatocytes. Hepatocyte Ultrastructure The organization and structure of the hepatocyte organelles permit the liver to carry out its metabolic functions. Mitochondria occupy approximately 18% of the liver cell by volume and participate in oxidative phosphorylation and the oxidation of fatty acids. Lysosomes catabolize endogenous substances as well as some exogenous wastes. Multivesicular bodies contain large amounts of protein derived from plasma. Microtubules and associated mechanotransducers may regulate the direction of vesicular transport in the cell. The liver is unique in that both the smooth and rough endoplasmic reticulum are well developed. As many as 50 Golgi complexes occupy a hepatocyte. The smooth and rough endoplasmic reticulum and Golgi complexes are collectively known as the liver microsomal fraction. The liver microsomes are known to participate in glycogenolysis; synthesis of cholesterol and bile salts; esterification of free fatty acids to triglycerides; synthesis of albumin, fibrinogen, and other proteins destined for export to the plasma; and glucuronidation of bilirubin, drugs, and bile salts. FUNCTION The liver has an extraordinary spectrum of functions. The organ regulates a massive amount of energy; stores, distributes, and disposes of various nutrients; and synthesizes, transforms, and metabolizes many endogenous substrates and pollutants. Over the past 5 years, major advances have occurred in molecular and cellular biology and immunobiology, including increased knowledge of mechanisms of signal transduction, growth control and cell death, and membrane physiology. This section capsulizes some of this fundamental biology and practical knowledge that influences our understanding of liver function and surgical disease. Energy Most of the body's metabolic needs are regulated in some way by the liver. To accomplish this, the liver expends approximately 20% of the body's energy and consumes 20% to 25% of the total utilized oxygen, despite constituting only 4% to 5% of the total body weight. The liver architecture seems specific for these demands. The blood supply includes the portal system between the intestinal and hepatic capillary bed, which helps establish a remarkably efficient extrahepatic circulation. The acinar unit permits each cell to be bathed by sinusoidal blood and at the same time separates a biliary compartment within a portion of its membrane to ensure an excretory pathway. The hepatocellular organelles in plasma membranes permit specific functions and, at the same time, interrelate with an extracellular matrix, which facilitates metabolic exchange between blood and hepatocytes. The liver not only conducts a large number of functions but also manufactures a large number of substances, such as plasma proteins, carnitine, and creatine, which service solely other organs or tissues. The liver collects and transforms such substrates to meet the fuel requirements of other tissues in response to various metabolic signals. It is the only organ that produces acetoacetate for use by muscle, brain, and kidney but not itself. The liver also uses little glucose for its own requirements and expends little of the energy generated by degradation of glycogen. It uses fatty acids that originate from the diet and fat, but also easily makes fatty acids as triacylglycerols and phospholipids, which are exported. The liver has a special capacity for gluconeogenesis from alanine arising in muscle but has little capacity for transamination of leucine, isoleucine, and valine. The energy-related functions of the liver are remarkably regulated by hormones, other agonists, and substrates coming to and from the liver. No doubt there are many other signals that regulate these exchanges. The contribution of this energy metabolism to the body's overall acid-base balance must be great, but surprisingly undiscovered are the signaling responses so important in this regulation. Functional Heterogeneity and Sinusoidal Membrane Traffic Under light microscopy, liver cells look basically the same. However, the cells from different acinar zones behave differently. In fact, many of the markers on the cell surfaces are different depending on the zone, or even depth within a segment. Krebs cycle enzymes are found highest in concentration in zone 1, whereas glutamine synthetase is highest in zone 3. The drug-metabolizing P-450 enzymes are concentrated in zone 3, particularly after enzyme induction by phenobarbital. The hepatocytes in zone 1, as expected, are more important for bile salt–dependent bile formation, because they are the first to come into contact with and readily absorb the detergents. In contrast, the hepatocytes in zone 3 are more important to bile salt–independent bile formation. The differences relate not only to blood flow but also to gene transcription rates. Receptor-mediated endocytosis is responsible for the transfer of large molecules, such as growth factors and carrier proteins. When they become occupied, the receptors on the sinusoidal membrane cluster into a pit when endocytosis begins. Sinusoidal plasma membrane is particularly receptor rich and metabolically important. A lateral domain, important in cell-cell interaction, separates it from the bile canaliculus. Endothelial cells and perisinusoidal cells patrol the sinusoids and have an increasingly recognized diverse set of functions, including protection, immune surveillance, and regulation of some major hepatic processes, including regeneration. Perisinusoidal cells consist of the Kupffer's cells so important in phagocytosis and antigen presentation, the fat-storing cells, or Ito cells, important in collagen metabolism and storage of vitamin A, and the rare pit cells that have natural killer cell and neuroendocrine activities. The sinusoidal cells also appear to be important in the production of growth-regulating molecules. Blood Flow The liver receives blood from the arterial and portal circulation; processes nutrients and metabolizes toxins and wastes; and stores, transforms, and distributes them to the vascular, biliary, or lymphatic circulations. Mean total hepatic blood flow has been estimated to be 100 to 130 ml. per kg. per minute. Seventy per cent to 75% of total hepatic blood flow comes from the portal vein, while the remainder comes from the hepatic artery. There is a reciprocal increase in hepatic arterial flow in response to a reduction in the portal flow, but the converse does not occur; portocaval shunt or ligation of the superior mesenteric artery results in an almost 100% increase in hepatic arterial flow. The compensation is not complete, however, so total hepatic flow does not return to normal. Reflex neural control and autoregulation appears to be an important regulatory factor for hepatic arterial flow but not for portal venous flow. To a large extent, portal venous flow into the liver is regulated by extrahepatic factors such as the rate of flow from the intestines and spleen. Food, bile salts, secretin, cholecystokinin, pentagastrin, epinephrine, vasoactive intestinal peptide, glucagon, and isoproterenol all increase portal blood flow. Although it is conceivable that flow would increase based on nutritional status, because of hepatic arterial flow regulation the total flow does not vary with metabolic state of the organism. Both intrinsic and extrinsic factors appear to be important in controlling the variable arterial blood flow. Intrinsic flow regulation occurs through arterial autoregulation based on the local concentration of adenosine surrounding the hepatic arteriole and portal venule. Adenosine is a potent arteriolar dilator; an increase in portal flow washes out perivascular adenosine and results in constriction of the hepatic arteriole, thereby maintaining a constant level of hepatic blood flow. Less is known about the extrinsic control mechanisms. Possible humoral regulators of extrinsic regulation include gastrin, glucagon, secretin, and bile salts. The hepatic artery is also densely innervated by sympathetic nerves and constricts in response to alpha-adrenergic receptor stimulation. Hepatic arterial flow and pressures reflect the systemic system. Portal blood flow appears to be controlled by resistance across a distinct hepatic venous– like zone. In normal liver there is no resistance attributable to either the portal venule or sinusoid. In experimental models, hepatic venous sphincters appear to contract in response to histamine, norepinephrine, and angiotensin. Portal venous pressures range from 7 to 10 mm. Hg, while sinusoidal pressures are 2 to 4 mm. Hg above the pressure in the inferior vena cava. Intrinsic liver flow is to a degree comparable to a sponge, but with various methods of regulation. The microvasculature is regulated by a series of sinusoidal inlet and outlet sphincters. Hepatic inflow increases with expiration and decreases with inspiration, which is opposite to the phasic flow in the vena cava. During vigorous exercise, total hepatic blood flow is decreased because of shunting of blood to muscle and the brain. The liver serves as a physiologic reservoir of blood: 25% to 30% of its volume is composed of blood, and during acute blood losses, 300 ml. or more can be released into the systemic circulation without adverse effects on liver function. Conversely, in the face of right-sided heart failure, up to 1000 ml. of blood can be stored in the liver without affecting liver function. Bile Formation Bile secretion is an active process, relatively independent of total liver blood flow, except in conditions of shock. Bile is formed at two sites: (1) the canalicular membrane of the hepatocyte and (2) the bile ductules or ducts . Total unstimulated bile flow in a 70-kg. man has been estimated to be 0.41 to 0.43 ml. per minute. Eighty percent of the total daily production of bile (1500 ml.) is secreted by hepatocytes and 20% is secreted by the bile duct epithelial cells. The principal organic compounds in bile are the conjugated bile acids, cholesterol, phospholipids, and protein. Because of the excellent correlation between bile acid output and bile flow, the term bile acid–dependent flow is used to describe this fraction of bile formation: bile flow is linearly related to bile acid output. Secretion of cholesterol and phospholipid is closely linked to the output of bile acids, except under certain conditions such as insulin or glucagon stimulation. The bile canaliculus is a long narrow channel that begins as a space of approximately 1 mm. in diameter, bounded by two or three hepatocyte canalicular membranes. It has no wall of its own, but the membrane has numerous microvilli. Canalicular flow may be generated in near absence of bile acids or during stabilized bile acid–dependent flow, and this is termed the bile acid–independent canalicular fraction. Abundant evidence exists now, suggesting that this independent fraction of bile may actually be controlled by hepatic bile production. As bile passes through the biliary ductules or ducts it is modified by secretion or absorption of epithelial cells. The highest cells in the biliary ductules have functions and architecture in common with both hepatocytes and ductular cells and so are called cholangiocytes. Secretion from biliary epithelial cells (canalicular bile) appears to be dependent on chloride channel stimulation. The best characterized hormone stimulator is secretin. Extracellular signaling molecules may control ion channels on the apical surface of the bile duct epithelial cells. The presence of such signals suggests an origin upstream of the canalicular membrane. These molecules may provide alternate methods to stimulate canalicular bile flow in conditions such as bile stasis or secretin-associated chloride channel defects (such as cystic fibrosis). Cholangiocytes contain receptors for epidermal growth factor, secretin, and somatostatin. The only known function of the gallbladder is to concentrate and store bile during fasting. Approximately 90% of the water in gallbladder bile is absorbed in 4 hours. Bile acids within the gallbladder may reach 50 times their concentration in hepatic bile. Cholecystokinin appears to be the principal physiologic stimulator of gallbladder concentration. It is released from the mucosa of the proximal intestine in response to food and simultaneously relaxes the sphincter of Oddi. Other peptides, such as vasoactive intestinal peptide, neuropeptide Y, motilin, histamine, prostaglandins, pancreatic polypeptide, and somatostatin, may be involved in control of gallbladder storage and emptying. Cholinergic stimulation causes contraction of the gallbladder and relaxation of the sphincter of Oddi. Bile acids in the intestine appear to have a negative feedback effect on release of cholecystokinin from the intestine. The importance of gallbladder function is debatable because humans have no nutritional consequences after cholecystectomy. Bile is a micellar solution . Sodium is the most important cation involved in canalicular secretion, while chloride plays an important role at the ductular level. Osmolality is approximately 300 mOsm per kg., which is isosmotic with plasma. The low osmolality reflects in part the aggregation of bile acids in micelles and consequent reduction of osmotic activity. The concentrations of the major electrolytes resemble those of lactated Ringer's solution; thus, lactated Ringer's solution is the fluid of choice for replacement of fluid lost through a biliary fistula. A prolonged biliary fistula results in impaired lipid and lipid-soluble vitamin absorption. These effects, for the most part, are reversed by replacement of bile into the upper gastrointestinal tract. Surprisingly, most patients are able to drink their own bile when it is mixed with orange juice or other drinks. Bile secretory pressure is usually 10 to 20 cm. of saline. Maximal secretory pressure is 30 to 35 cm., even in the presence of complete biliary obstruction. Numerous proteins are present in physiologic bile; the most abundant is IgA. The presence of IgA depends on an active secretory process, whereas that of albumin does not. It is likely that IgA is important to the immunocompetence of the gastrointestinal tract. Enterohepatic Circulation Bile salts secreted into the biliary system empty into the intestine, where they are efficiently absorbed into the enterohepatic circulation . The liver extracts the bile acids and transports them back to the canalicular membrane where they are resecreted back into the biliary system. This process is referred to as the enterohepatic circulation. Total bile pool size in humans is 2 to 5 gm. and undergoes this circulation 2 to 3 times per meal and 6 to 10 times a day, depending on dietary habits. In addition, 0.2 to 0.6 gm. is lost in the stool per day, and this quantity is replaced with newly synthesized bile acids. Therefore, 20 to 40 times more bile acid than is normally synthesized is delivered into the intestine, underscoring the importance of this circulation. Under normal circumstances, serum bile acid levels are low (~5 mmol.) because of the 95% extraction efficiency of the liver: the liver can remove 80% of the bile acids delivered to it in a single pass. Serum bile acid levels are frequently increased in a patient with liver failure. The active secretion of bile acids across the canalicular membrane is the primary metabolic pump of the enterohepatic circulation. In discussing the enterohepatic circulation, it is useful to distinguish between primary and secondary bile acids. Primary bile acids are synthesized from cholesterol by the liver and consist of cholic acid and chenodeoxycholic acid in humans. Secondary bile acids are formed in the intestinal lumen by bacterial dehydroxylation and consist of deoxycholic and lithocholic acid from cholic acid and chenodeoxycholic acid, respectively. Essentially all primary and secondary bile acids are conjugated with the amino acids glycine and taurine. Amino acid conjugation lowers the pKa of bile acids so they remain ionized in the intestinal lumen and are not passively absorbed through nonionic diffusion. Conjugated bile acids also form micelles, which more effectively facilitate lipid digestion and absorption from the small intestine. Various intestinal problems such as regional enteritis, ileal resection, Zollinger-Ellison syndrome, radiation enteritis, and blind loop syndrome may be associated with deficient bile acid absorption, which leads to diarrhea, steatorrhea, or vitamin B 12 deficiency. A decreased bile acid pool may also predispose to the formation of gallstones. In blood, bile salts are tightly bound to both serum albumin and lipoproteins, particularly high-density lipoprotein. Hepatic uptake is carrier mediated and follows Michaelis-Menten kinetics. Bilirubin Metabolism Bilirubin, a breakdown product of heme, is excreted almost entirely in the bile. With hepatocellular disease or extrahepatic biliary obstruction, free bilirubin may accumulate in blood and tissues. Approximately 75% of bilirubin is derived from senescent red blood cells. Bilirubin circulates bound to albumin, which protects tissue from its toxicity. It is rapidly removed from the plasma by the liver through a carrier-mediated transport system. In the hepatocyte, bilirubin is bound to other proteins (Y and Z), which probably have a role in transport. It is conjugated with glucuronide and secreted in bile. Conjugated bilirubin may form a covalent bond with albumin; this is called delta bilirubin. The implications of delta bilirubin are still being investigated. Disorders of bilirubin metabolism leading to predominantly unconjugated hyperbilirubinemia include neonatal hyperbilirubinemia, Crigler-Najjar Type I (which usually leads to kernicterus and death), the more benign Crigler-Najjar Type II, and Gilbert's syndrome. Disorders characterized by predominantly conjugated hyperbilirubinemia include Dubin-Johnson syndrome, Rotor's syndrome, and recurrent intrahepatic cholestasis; patients with these disorders usually have a benign course. In the intestine, bilirubin is reduced by bacteria to mesobilirubinogen and stercobilinogen, collectively termed urobilinogen. These are both excreted in the stool. A fraction of urobilinogen is oxidized to urobilin, which is brown pigment and gives stool its normal color. Part of urobilinogen is resorbed in the intestine and excreted in the urine. With complete biliary obstruction, urobilinogen cannot form and, therefore, will not appear in the urine. Carbohydrate Metabolism The liver has a central role in energy metabolism: it helps provide a continuous source of glucose for the central nervous system and red blood cells. During the fed state, results of intestinal carbohydrate digestion (glucose: 80%; and galactose and fructose: 20%) are delivered to the liver. The latter two are rapidly converted to glucose. Glucose absorbed by the hepatocyte is converted directly to glycogen for storage up to a maximum of 65 gm. of glycogen per kg. of liver mass. Excess glucose is converted to fat. Glycogen is also produced by muscle, but this is not available for use by any other tissues. During the fasting state, this glycogen is the primary source of glucose. However, after 48 hours of fasting, liver glycogen is exhausted, and proteins mobilized primarily from muscle, mainly alanine, are converted by the liver to glucose. Lactate produced by anaerobic metabolism is metabolized only in the liver. Ordinarily it is converted to pyruvate and subsequently back to glucose. This shuttling of glucose and lactate between liver and peripheral tissue is carried out in the Cori cycle. The brain does not participate in this cycle, and a continuous source of glucose for the brain must come at the expense of muscle proteins. In liver disease, the metabolism of glucose is often deranged. Frequently, in patients with cirrhosis, the portosystemic shunting causes decreased exposure of portal blood to the hepatocytes, producing an abnormal result of the oral glucose tolerance test. Hypoglycemia is rare in chronic liver disease, since the synthetic capacity of hepatocytes is preserved until late in the disorder. In fulminant hepatic failure, however, there is extensive loss of hepatocyte mass and function, and hypoglycemia supervenes as gluconeogenesis fails. Lipid Metabolism There are three sources of free fatty acid available to the liver: fats absorbed from the gut, fat liberated from adipocytes in response to lipolysis, and fatty acids synthesized from carbohydrates and amino acids. These fatty acids are esterified with glycerol to form triglyceride. The export of triglycerides is dependent on the synthesis of very low density lipoproteins. In cases of excess supply of fatty acid, there is lipid accumulation in the liver because there is an imbalance of triglyceride relative to very low density lipoproteins. This is seen in obesity, corticosteroid use, pregnancy, diabetes, and total parenteral nutrition. Simple protein malnutrition or protein-calorie imbalance may also result in fatty change of liver, based on decreased export of triglycerides, because of limited supply of precursors for hepatic synthesis of lipoproteins. The fatty infiltration of alcohol abuse is the result of several abnormalities: (1) alcohol is a source of calories, which are converted to acetyl-coenzyme A, which is a substrate for fat synthesis; (2) the reduced form of nicotinamide-adenine dinucleotide produced in the metabolism of alcohol inhibits fatty acid oxidation and shifts metabolism toward triglyceride synthesis and esterification; and (3) chronic alcoholism may, because of malnutrition, inhibit synthesis of very low density lipoproteins. The liver also has a central role in cholesterol metabolism. It is the most active site of cholesterol and bile salt synthesis. In mammals, 90% of cholesterol is synthesized de novo from its precursor acetyl-coenzyme A. Hydroxymethylglutaryl coenzyme A reductase is the rate-limiting enzyme of cholesterol metabolism. A competitive antagonist, mevinolin, can block this enzyme and effectively lower plasma cholesterol. Cholesterol can enter the liver cell with four different species of lipoprotein: chylomicrons, very low density lipoproteins, low density lipoproteins, and high density lipoproteins. The major carriers of cholesterol are low density lipoproteins. Bile salt synthesis is the major catabolic pathway of cholesterol in liver, and 7-alpha-hydroxylase is the rate-limiting enzyme for the conversion of cholesterol to bile acids. The other important route of cholesterol elimination is direct secretion into the bile. Protein Metabolism Hepatic protein synthesis and catabolism are vitally important. At least 17 of the major human plasma proteins are synthesized and secreted by the liver. The liver is the only organ that produces serum albumin and alpha-globulin, and it synthesizes most of the urea in the body. Production of various serum proteins is an important index of liver function. Albumin is the most abundant serum protein, its synthesis accounting for 11% to 15% of total hepatic synthesis. Synthesis of albumin is influenced by nutritional status, thyroxine, insulin, glucagon, cortisol, and cytokines produced in the systemic inflammatory response. Dramatic changes in both type and amount of plasma protein produced by the liver take place in systemic inflammatory states. The acute-phase reactants produced by the liver in response to interleukin-6 increase in a sudden transient rise in production of several proteins. These include C-reactive protein, serum amyloid A, and fibrinogen. Albumin synthesis decreases. The acute-phase proteins have a wide range of biologic activities, including inhibition of proteases, blood clotting, opsonization of bacteria and debris, modulation of the immune response, and binding of heavy metals. In general, it is thought that the acute-phase reactants localize and limit the tissue damage while enhancing microbial clearance. Vitamin Metabolism The liver has many important roles in the uptake, storage, and mobilization of vitamins. Most important are the fat-soluble vitamins A, D, E, and K. The absorption of these is dependent on bile salts. The vitamins appear in the thoracic duct 2 to 6 hours after oral administration. Vitamin A is exclusively stored in the liver, and excessive ingestion of vitamin A may be associated with significant liver injury. A role for the storage of vitamin A in Ito cells has been suggested. The initial step in vitamin D activation occurs in the liver, where vitamin D 3 is converted to 25-hydroxycholecalciferol. Of particular surgical significance was the discovery of vitamin K. This vitamin is essential for the gamma-carboxylation of the vitamin K–dependent coagulation factors II, VII, IX, and X. These factors are inactive without gamma carboxylation. In 1929, Dam observed that chicks developed hemorrhages when fed fat-free diets. He designated the active ingredient in normal feed as vitamin K, after the German word Koagulation. The veterinary hematologic disorder called sweet clover disease, first reported in 1922 in cattle that developed fatal hemorrhages, was later found to be caused by bis-hydroxycoumarin, a vitamin K antagonist. The minimal daily requirement of vitamin K is small, less than 0.1 mg. per kg. of body mass. Coagulation The liver synthesizes 11 proteins critical for hemostasis; all the procoagulant factors except von Willebrand's factor are produced by the liver. Factors I, VII, IX, and X share the unique characteristic of having gamma carboxyl glutamic acid in their amino acid sequence and are dependent on vitamin K for activation; without vitamin K, they do not undergo gamma-carboxylation and are inactive. Warfarin (Coumadin) impairs the formation of vitamin K–dependent factors by reducing the amount of vitamin K available to participate in gamma carboxylation of these factors. Clinically, warfarin is followed by observing the prothrombin time. Reversal of the effect of warfarin occurs by parenteral administration of vitamin K or coagulation factors. Classically, there are two pathways for fibrin formation (intrinsic and extrinsic); physiologically, it is more likely that the two act in concert. The extrinsic pathway causes large amounts of clot to be formed in seconds and is limited only by the amount of tissue thromboplastin released. The intrinsic pathway requires several minutes to form a clot and can be blocked by a number of inhibitors. In clinical practice, the extrinsic pathway is monitored by measuring the prothrombin time, whereas the intrinsic pathway is monitored by measuring the partial thromboplastin time or accelerated partial thromboplastin time. Factor VII has the shortest half-life (5 to 7 hours), and in patients with hepatic dysfunction the synthetic ability of the liver may be assessed by monitoring the prothrombin time, because this test is dependent on adequate amounts of functional factor VII. Metabolism of Drugs and Toxins Drug and toxin metabolism is primarily a hepatic function. The range of metabolic transformations that foreign compounds undergo is conveniently categorized into two broad headings: (1) the phase I reactions of oxidation, reduction, and hydrolysis and (2) the phase II reactions, in which a compound is combined with an endogenous molecule to form a conjugate. Oxidative reactions represent most of the phase I biotransformations in what has come to be known as the cytochrome P-450 system. Regeneration Clinically, the regenerative capacity of the liver is well known and typically triggered by partial hepatectomy. During the midlife of humans, the liver will regenerate to a volume of 25 ± 1.2 ml. per kg. However, the exact time course for the regenerative phase in humans is lacking. Van Thiel and associates have previously presented clinical data on two patients receiving livers from donors an average of 10 kg. smaller than the recipients, which resulted in livers 29% and 59% smaller than expected had the donor liver and recipient been ideally matched. Serial computed tomographic scans after transplantation revealed an average increase of 70 ml. per day of liver volume, until achievement of a liver volume consistent with that expected for the recipient's size, age, or sex was achieved. Interestingly, whereas plasma levels of amino acids, glucagon, and insulin and standard liver injury tests have been monitored, none has correlated with changes in graft size. Most of these are generally believed to be poor indices of the hepatocyte proliferation, and there is still no specific clinical marker for hepatocyte proliferation. Timetables of test results during regeneration suggest that bilirubin levels return to normal at 3 weeks and albumin levels normalize after 5 weeks. Potential growth promoters include prostaglandins, platelet-derived growth factor, epidermal growth factor, hepatocyte growth factor (released from platelets by thrombin), and epinephrine. To date, the most important negative growth factors are tumor growth factor-beta and age. The regenerative capacity of the hepatocytes may be determined by factors other than the hepatocytes themselves. This inference comes from studies of reduced-size liver grafts from related donors, in which the transplanted livers regenerated faster than the residual liver in the donor. These observations substantiate previous findings that regeneration is determined by an intrinsic liver/host body:volume ratio, independent of functional liver mass. Further investigations are necessary to characterize more fully the regenerative response of the liver. Future Developments A number of areas of active research are likely to develop new concepts with respect to disease and therapy. Advances are likely in the following fields: (1) characterization of liver stem cells, (2) methods of molecular modification of the genome, (3) hepatocyte transplantation or replacement, and (4) apoptosis. Studies of liver development have shown that committed endodermal cells form hepatoblasts that have dual lineage progenitor capacity and give rise to mature hepatocytes, intrahepatic bile ducts, and portions of the extrahepatic ducts. Certain chemicals induce the proliferation of oval cells, which may play some role in carcinogenesis. Considerable research is ongoing with respect to permissive conditions for hepatocyte engraftment and survival. Transplantation of hepatocytes in ectopic sites has led to some enthusiasm with respect to their use in acute liver failure and also in methods of altering the genes of cells for better integration into the host. Gene therapy seems to have a great deal of potential applicability to the liver. This research is divided into two general strategies, somatic cell and germ cell therapy (i.e., introduction of foreign genes into nongerm cell versus germ cell lines). The strategies can be further subdivided into gene replacement or augmentation strategies, ex vivo and in vivo. Some progress has been reported by using viral vectors or other carriers, and by targeted delivery of polynucleotides to inhibit gene expression. Apoptosis, or DNA-encoded cell death, appears to play an important role in the cessation of liver regeneration. Early validation of this concept in the liver has led to a great flurry of research relating apoptosis to benign or malignant conditions. Assessment of Liver Function Although there is no magic test, a number of methods are useful in the diagnostic evaluation of the patient with potential liver disease. In this section are mentioned only some routine tests of liver function and several quantitative estimates of liver function. Routine Tests Routine tests of liver function include the liver transaminases, which are named either by the products of the reaction (i.e., serum glutamic oxaloacetic transaminase [SGOT] and serum glutamic pyruvic transaminase [SGPT]) or by the amino group donor (i.e., aspartate transaminase [AST] or alanine transaminase [ALT]); alkaline phosphatase (ALP); gammaglutamyl transpeptidase (GGT); leucine aminopeptidase (LAP); 5'-nucleotidase; serum albumin and transferrin; and serum lipids and lipoproteins. Liver Transaminases. Increased liver transaminase levels in liver disease reflect leakage from injured liver cells. The degree of elevation of the transaminases generally reflects the severity of hepatic necrosis, except in the important setting of alcoholic hepatitis, when levels seldom exceed 200 to 300 I.U. per liter. Alkaline Phosphatase Activity. Activity of ALP is detected in many tissues, including the liver, bile ducts, intestines, bone, kidneys, placenta, or white blood cells. The serum ALP level is also elevated in a number of conditions not associated with hepatobiliary disease, such as pregnancy, normal growth, bone tumors, and liver tumors (the Regan isoenzyme). The reason for ALP elevation is apparent in most cases. When the reason is not apparent, several methods, such as electrophoresis and relative heat stability, differentiate the hepatobiliary enzyme from other isoenzymes. The most available solution in the clinical setting is to order a 5'-nucleotidase, LAP, or GGT activity measurement. These generally parallel ALP levels in hepatobiliary disease. Increased serum hepatobiliary ALP activity indicates bile duct obstruction, parenchymal disease, infiltrative lesions of the liver, or repair after hepatocyte injury. Increased ALP activity reflects both increased enzyme synthesis and altered biliary excretion or leakage from damaged cells. The highest levels of ALP occur, in general, with extrahepatic bile duct obstruction, followed by intrahepatic cholestasis and then neoplasms of the liver. The degree of ALP elevation is a predictor of the degree of liver involvement or metastatic disease. Other Enzymes. LAP is a ubiquitous cellular peptidase, and 5'-nucleotidase is a plasma membrane enzyme. GGT is present in many tissues, and its elevation suggests hepatobiliary disease, myocardial infarction, or pancreatic or neuromuscular disease. GGT also monitors the degree of ingestion of alcohol. Albumin. Albumin is a useful clinical marker of synthetic function in chronic hepatic insufficiency. It is synthesized only in the liver, and its level in the blood is determined by liver function, nutritional state, thyroid hormone, or adrenal corticosteroids. The normal rate of albumin synthesis is 110 to 200 mg. per kg. per day. The exact mechanisms involved in albumin metabolism in the healthy state are not clear. Albumin loss is augmented in certain disease states, such as burns, sepsis, nephrotic syndrome, and proteinlosing enteropathies. When other mechanisms of albumin loss are excluded, the serum albumin level is an accurate marker of liver failure or altered hepatic function. Unfortunately, because of its long half-life, albumin measures just chronic and not acute liver failure. Transferrin. Also synthesized in the liver, transferrin has a much shorter half-life than albumin. Changes in transferrin levels reflect more acute changes in liver function than do changes in albumin levels. Lipid and lipoprotein electrophoreses also change in acute or chronic liver disease, owing to abnormal or altered synthesis. Much work has gone into investigating these patterns, but their measurement has not yet proved particularly useful clinically. Specific Tests. Specific tests of liver disease include screening tests for hepatitis and for other benign chronic liver disease. Such tests include antimitochondrial (primary biliary cirrhosis), smooth muscle (sclerosing cholangitis), antinuclear antibody, alpha 1-antitrypsin (antitrypsin deficiency), blood alcohol, plasma ceruloplasmin, or amylase levels. Specific markers for neoplasms include alpha-fetoprotein and carcinoembryonic antigen levels. Infectious serologic markers include cytomegalovirus, Epstein-Barr virus antibodies, leptospiral agglutination, fasciola, ameba, hydatid complement fixation tests, and the Wasserman reaction. Quantitative Assessment Bromsulphalein and Indocyanine Green. These dyes are removed from the circulation by the liver. Such intravenous tests have been used to assess liver dysfunction in the absence of jaundice. Each is a measure of biliary excretion and is a more specific quantitative test than any of the routine tests of liver function or cholestasis. However, neither has particular clinical utility, and bromsulphalein has a long history of being put on and taken off the commercial market. Galactose Elimination Capacity. This test reflects hepatocellular function but requires multiple determinations over a 2-hour period. Galactose is safe and injected intravenously at a dose that saturates the enzyme system responsible for its elimination. The preliminary step is the initial phosphorylation by galactokinase. Aminopyrine Breath Test. Aminopyrine as well as caffeine have been used as breath test substances that measure the efficiency of the cytochrome P-450 (microsomal) system where they are metabolized. Aminopyrine is labeled with carbon-14 and given by mouth. Carbon dioxide samples labeled with carbon-14 are collected at intervals over 2 hours. This test reflects the residual functional microsomal mass and, thus, viable hepatic tissue. It has more value in assessing prognosis than for screening. Serial salivary caffeine clearance is a similar measure. Antipyrine clearance can also be measured, but the test requires over 30 hours. Lidocaine Metabolite Formation (MEGX Test). Lidocaine similarly is metabolized by oxidative N-demethylation by the cytochrome P-450 system. Monoethylglycinexylidide (MEGX) is formed correlating with lidocaine clearance. Clearance 15 minutes after injection gives a quantitative assessment of liver function, which may correlate with graft survival before liver transplantation. Tests of Coagulation. The prothrombin time is in general a sensitive marker of the severity of liver failure. Prolongation indicates deficiency not only of the thrombin complex but also of factors XI and XII. Estimation of individual clotting factors is rarely required. However, some evidence suggests that a factor V concentration of less than 10% on admission in acetaminophen-induced acute liver failure predicts a poor outcome. The ratio of factor VIII to factor V may also be valuable. Prognostic significance of several other clotting factors relating to various kinds of fulminant failure is receiving intensive scrutiny. II. PYOGENIC AND AMEBIC LIVER ABSCESS PYOGENIC AND AMEBIC LIVER ABSCESS Liver abscess remains a formidable diagnostic and therapeutic problem, but significant strides in management have occurred over the past two decades. Changing etiologies of liver abscess reflect both improvements in health care and increased recognition of the condition in sicker, often immunocompromised patients. Pyogenic (bacterial) and amebic abscesses share many clinical features and are therefore discussed together. The pyogenic type is much more common in most sections of the United States; yet amebic abscess is endemic in many areas of the world and requires clinical suspicion for correct diagnosis. Fungi, cytomegaloviruses, and other organisms also cause liver abscess, predominantly in the immunocompromised host, but are less common and usually cause more diffuse hepatic disease. Echinococcosis generally has a different clinical presentation from pyogenic or amebic abscess, unless there is secondary bacterial involvement. Liver abscess has been recognized since Hippocrates (circa 400 B.C.), who speculated that prognosis is related to the type of fluid within the lesion. 21 In the early nineteenth century, Bright 6 suggested that amebae might contribute to the formation of hepatic abscess, and Koch, in 1883, described amebae in the wall of a hepatic abscess. Fitz 10 and Dieulafoy 9 both emphasized the importance of intra-abdominal (bacterial) sources of infection in the pathogenesis of the disease. Dieulafoy 9 coined the term la foie appendiculaire in describing multiple hepatic abscesses subsequent to perforated appendicitis with pyelophlebitis. Ochsner and DeBakey provided classic treatises on pyogenic and amebic hepatic abscess in 1938 and 1943. The latter authors reviewed a large personal experience and the world literature and emphasized the similarity in clinical presentation between the two types of abscesses. Although clinical signs have remained the same, radiologic methods of diagnosis have greatly improved, and it is rare that a lesion is overlooked when ultrasonography or computed tomography (CT) is used. Antibiotic therapy has also improved, but the principal advance in the management of hepatic abscess has been the application of percutaneous aspiration or of laparoscopic techniques for diagnosis or treatment. INCIDENCE The overall incidence of liver abscess remains relatively stable, although the distribution of causes is changing. Pyogenic abscess represents approximately 80% of the cases in the United States, and superinfection represents another 10%. Amebae are the primary cause of 10%, and fungi and other organisms cause less than 10%. The overall rate of pyogenic abscess in the United States is estimated to be between 8 and 15 per 100,000 population. The incidence of both pyogenic and amebic abscess is higher in nations where medical care is not immediately available. For example, in Malaysia, pyogenic abscess constitutes 0.85% of total hospital admissions. Amebic infestation is higher in countries in tropical or subtropical zones, locations with poor sanitation, and mental institutions. In the United States in 1975, the Center for Disease Control (CDC) noted 1.3 cases of amebiasis reported per 100,000 population. The CDC recorded 3500 cases of amebiasis per year, data that probably underestimate the actual number of cases. Immigrants and tourists from developing countries have a higher incidence, and American tourists to tropical areas are more likely to develop invasive amebiasis than permanent residents. Amebae are estimated to infest 15% to 30% of the population of Mexico, Africa, Southeast Asia, and South America. Local inhabitants are less likely than visitors to manifest symptoms, presumably due to partial immunity. Hepatic abscess is the most common extraintestinal manifestation of amebiasis. Hepatic amebiasis is reported in 3% to 10% of afflicted patients. DeBakey and Ochsner found an average incidence of hepatic involvement associated with intestinal disease to be 13.2%. In certain severely infested locations up to a 40% incidence of hepatic involvement is reported. Liver abscess of both types affects both sexes and all age groups. In recent years, pyogenic abscess affected slightly more males than females, which is consistent with earlier series. The 40- to 60-year-old age group is the most commonly afflicted, and children represent a distinct group because of a higher rate of immunosuppression in hospitalized youth. In a series from Duke University Medical Center all six patients with pyogenic abscess who were younger than 15 years of age were male. Over the past several years in Los Angeles, the male-to-female ratio has been nearly 2:1 in young patients. This changing sex distribution in younger patients probably reflects the impact of the acquired immunodeficiency syndrome (AIDS). Amebic abscess affects males more than females in as much as a 9 to 10:1 ratio. In general the patients are younger, with the highest incidence in the 20- to 50-year-old age group. Several recent reports indicate a marked increase in the incidence of amebic liver abscess in children younger than the age of 3. There does not appear to be any particular racial susceptibility except for that related to living conditions. Examples include Mexican-Americans in the American Southwest and the predisposition of South African blacks compared with South African whites. PATHOGENESIS The pathophysiology of liver abscess in general or pyogenic abscess in particular involves two basic elements: the presence of the organism and the vulnerability of the liver. The spread of bacterial or other organisms to the hepatic parenchyma may occur through (1) the portal system; (2) ascension from the biliary tree; (3) the hepatic artery during generalized septicemia; (4) direct extension from subhepatic or subdiaphragmatic infection; or (5) a direct route following trauma . Most organisms enter the liver through the portal route. Hepatic clearance of portal bacteria is probably a very common event in healthy persons. The human liver remains sterile in most circumstances because of an efficient clearance mechanism that prevents colonization of hepatic sinusoids or parenchyma. Multiplication, tissue invasion, and abscess formation occur secondary to the introduction of other factors such as necrotic tissue, hepatic injury, malignant tumors, microemboli, poor perfusion, or congenital or acquired biliary or vascular obstruction. Pyogenic hepatic abscess usually represents an infective process in another organ. The process is usually within the abdomen. Appendicitis was by far the common source in most earlier series, representing 35% of the total group in Ochsner's patients. Appendicitis is involved in only approximately 10% of cases in more recent series The other most common sources are cholecystitis, biliary or pancreatic cancer with obstruction, diverticulitis, regional enteritis, trauma, generalized sepsis, and pelvic inflammatory disease. Patients receiving chemotherapy for hematologic or solid malignancies are at increased risk. Other important associations with hepatic abscess in adults include colon cancer, diabetes, and cardiopulmonary disease. Associated conditions in children are malignancy, non-AIDS immunodeficiency states, AIDS, polycystic disease, cholecystitis, necrotizing enterocolitis, and congenital hepatic fibrosis. The most common non-AIDS immunodeficiency states occur after transplantation, as a result of liver failure, after treatment of malignancy, or with chronic granulomatous disease of childhood. Despite advances in diagnostic techniques and an aggressive search for a source, no probable cause of hepatic abscess has been identified in 13% to 35% of cases since 1984. Cryptogenic abscesses constituted 22% of Ochsner's collected series in 1938. This incidence may increase further with more use of percutaneous drainage techniques because of fewer opportunities for abdominal exploration. In most previous series, cryptogenic abscesses were usually solitary, and the associated mortality was low. It is also possible that mortality associated with cryptogenic abscess could increase because of more undetected problems, such as underlying malignancy. Portal pyelophlebitis is an infection of the portal vein, usually manifested radiographically by air in the portal vein. Although portal venous infection undoubtedly occurs more frequently than the demonstration of portal venous air, the latter complication is usually lethal. Pyelophlebitis is a likely cause of hepatic abscess. In this condition, mesenteric veins at the site of an inflammatory process thrombose and subsequently extend into the portal system and embolize to the liver. The septic emboli block venous radicles, giving rise to polymorphonuclear leukocyte, lymphocyte, and macrophage migration to the region and an intense inflammatory reaction. Foci are usually multiple, but multiple sites may coalesce into a “solitary” abscess, with or without septation. Multiple, microscopic hepatic abscesses are often found at autopsy in patients dying of sepsis with portal pyelophlebitis. More abscesses occur in the right than in the left lobe. This is probably secondary to preferential laminar drainage of the superior mesenteric vein to the right lobe, although some controversy exists concerning this explanation. Abscess formation secondary to biliary obstruction follows a slightly different pattern, with similar results. After obstruction of bile ducts, bacteria multiply and ascend into intrahepatic biliary radicles and canaliculosinusoidal channels, producing cholangitis. Bacterial proliferation causes further biliary distention with lymphatic and portal invasion and formation of a pyelophlebitic abscess. Further coalescence allows escape of pus into surrounding hepatic tissue with formation of multiple pockets and subsequent septations of a predominant cavity. Biliary tract disease has supplanted appendicitis as the most common gastrointestinal problem associated with liver abscess. Bile ducts, biliary lymphatics, and periductal vascular channels are the primary routes by which abscesses develop in association with biliary infection. Liver abscess occurs most commonly with cholecystitis, choledocholithiasis, and malignant or benign biliary stricture. Malignant obstruction or metastatic adenocarcinoma causes more cases of liver abscess than observed previously because of improved palliative treatments, such as percutaneous or endoscopic biliary drainage techniques. Patients undergoing biliary-enteric bypass are at increased risk of abscess formation, even when there is no demonstrable stricture. Several studies suggest that choledochoduodenal anastomoses are associated with a high incidence of bacteremia or abscess than Roux-en-Y hepaticojejunostomy or choledochojejunostomy. A greater potential for reflux of foodstuffs in the former drainage procedure supports the latter observation. The incidence of a potential biliary source in association with liver abscess is estimated at 30% to 50%. Trauma predisposes hepatic parenchyma to abscess formation by several mechanisms: bile leakage, decreased perfusion, foci of hepatic necrosis, direct introduction of bacteria through penetrating high- or low-velocity missiles, and hematoma formation. The combination of bile leakage, hematoma, and necrotic tissue provides a rich culture medium for bacteria introduced by the trauma itself or entrapment of bacteria borne in blood or bile. Modern therapy for various tumors has introduced an important new etiology for pyogenic abscess—iatrogenic cell death. Specific therapies relatively commonly associated with hepatic abscess include chemoembolization, alcohol injection, or cryoablation of hepatic tumors. In one report from the National Cancer Institute, bacterial abscesses were more common in older patients with solid tumors and invasive procedures; in contrast, fungal abscesses occurred more in younger patients with leukemia or lymphoma after chemotherapy and resultant neutropenia. PATHOLOGY AND MICROBIOLOGY Pyogenic hepatic abscesses have some characteristic gross and microscopic pathologic features. In most series, right hepatic abscesses predominate by a nearly 3:1 ratio. The presumed explanation for this is streaming of the superior mesenteric vein fraction of portal flow to the right lobe of the liver as well as its relatively greater volume. Bilobar metastases occur in 1% of patients. Most series indicate a nearly equal distribution between solitary and multiple liver abscesses. Abscesses vary from less than a millimeter to several centimeters. They may appear honeycombed with multiple lesions, although this appearance is unusual except with fungal organisms. Interestingly, the size of solitary abscesses nearly tripled in one study between 1945 to 1957 and 1971 to 1983. Grossly, hepatic abscesses appear yellow, compared with the normal, deep maroon hepatic parenchyma that surrounds them. The organ is usually subtly enlarged, and palpation may reveal fluctuant areas corresponding to the pus-filled cavity. The liver is often adherent to surrounding organs or the diaphragm because of associated capsular inflammation. Small abscesses deep within the parenchyma rarely exhibit these findings. Most traumatic abscesses are solitary and localized near the site of injury. Microscopically, acute inflammatory reaction is observed with necrosis and hepatocyte cords in the portal triad regions, and cholestasis may be evident in adjacent tissue. Organisms recovered from liver abscesses vary greatly but generally reflect bile or enteric flora. Reasons for the variability include differences in antibiotics before culture, culture techniques, or patient populations. In most recent series, most patients have a positive culture, and over half harbor more than one organism. Solitary abscesses are more likely than multiple ones to grow multiple organisms. The high frequency of “sterile” abscesses found in earlier studies was probably due primarily to an inability to isolate anaerobic or microaerophilic organisms. At present, the most likely reason for a sterile culture is effective antibiotic therapy. The most common aerobic organisms in most series are Escherichia coli, Klebsiella, and Enterococcus. The most common anaerobes are Bacteroides, anaerobic streptococci, and Fusobacterium species. Streptococcal species (aerobic, anaerobic, or microaerophilic) are found in 25% to 30% of cultured abscesses and are believed to be of increasing importance in the pathogenesis of pyogenic abscess. The presence of isolated colonies of E. coli or Klebsiella should raise the suspicion of a biliary source, while the presence of anaerobes suggests a colonic source. One interesting observation is the more common appearance of staphylococcal abscesses in young males, compared with other patient groups. More than half of patients not receiving antibiotics may have microorganisms cultured from the peripheral blood. Amebic liver abscess follows intestinal infestation by Entamoeba histolytica. The most common mode of transmission of E. histolytica is by individual contact rather than contaminated drinking water or food. Venereal transmission usually causes genital, gut, or visceral disease. Two forms of the protozoan may be found in stool specimens: trophozoites and cysts. Trophozoites are the invasive form and are derived from cysts. The factors that determine clinically significant disease are poorly understood. The simple finding of trophozoites or cysts in the stool is so common that this alone is not evidence of active disease. The incidence of liver disease in patients with intestinal amebiasis is reported to be between 3% and 25%. There is a latency period of several weeks between intestinal infection and observable hepatic involvement. The amebae reach the liver through the portal vein. The trophozoites either degenerate in the portal venous radicles or migrate to an adjacent area, causing necrosis and liquefaction. The areas of destruction coalesce to form most commonly a single large cavity in the right lobe. The abscess may vary from less than 1 cm. to 25 cm. in diameter. The contents are a mixture of necrotic hepatic parenchyma and blood that yields a classic “anchovy paste” appearance. This is usually sterile and odorless. Secondary bacterial infection occurs in approximately 10% of cases and may change the color and odor of the contents. Protozoa are usually found only in a rim of necrotic tissue and are therefore unlikely to be aspirated. Seventy-five per cent to 90% of abscesses are found in the right lobe. Left lobe or bilobar involvement usually indicates more advanced disease. Right lobe lesions are more likely to rupture intraperitoneally, whereas left lobe lesions rupture into the pericardium or pleural space . As with pyogenic abscess, the liver is usually enlarged. Capsular adhesions to the diaphragm or adjacent tissue are generally not as numerous. Early or acute lesions have thin walls with little fibrosis, whereas older abscesses have a more well-formed fibrous capsule, sharply demarcating them from normal liver parenchyma. There is usually marked necrosis of hepatic parenchyma within the lesions, with little polymorphonuclear cell infiltration. As the abscess matures, it becomes spherical, and more eosinophilic staining debris develops. Calcification is unusual except in chronic abscesses. Like pyogenic hepatic abscess, the amebic variety may be associated with depressed immunologic states. Experimental infection is easier to induce after splenectomy, probably because of decreased macrophage clearance of the parasite. Antimacrophage serum exacerbates and bacille Calmette-Guérin improves host defenses against amebae. Cellmediated immunity is impaired during the first 2 weeks of infection in normal patients without treatment. Therefore, suppression of immune response during systemic infection may have a role in amebic liver abscess. DIAGNOSIS The diagnosis of hepatic abscess is challenging because clinical signs are usually not specific. Early differentiation between pyogenic and amebic liver abscess may be even more difficult because of the similarity in signs, symptoms, and radiologic features. Both pyogenic and amebic abscess should be considered lethal unless detected early. For pyogenic abscess, two diagnoses are necessary for optimal management: the abscess condition itself and the underlying source. Neither diagnosis may be apparent initially, but both can usually be discovered with appropriate evaluation. The diagnosis of hepatic abscess is apparent at the time of admission in a minority of patients but, if suspected, is readily diagnosed by ultrasonography or CT in over 95% of patients. The majority of patients with hepatic abscess have symptoms of less than 2 weeks' duration, although one third have been affected a month or longer. The primary symptoms are fever, malaise, chills, anorexia, weight loss, abdominal pain, and nausea. With amebic abscess, a recent diarrheal syndrome can be documented in only a minority of patients. An occasional historical feature is a long-standing respiratory infection occurring before the development of abdominal symptoms. Fever of unknown origin is the presentation in a significant number of patients. Rarely, patients with either amebic or pyogenic abscess are admitted with diffuse peritonitis, shock, or hepatic failure. Possible physical findings include right upper quadrant tenderness, pleural dullness to percussion, fever, hepatomegaly, and jaundice. Tenderness may be elicited by either direct palpation or percussion of the right rib cage. Most patients have leukocytosis and some liver enzyme abnormality. The most common findings on plain abdominal or chest radiographs are rightsided atelectasis, an elevated hemidiaphragm, pleural effusion, or pneumonia. Occasionally, a subdiaphragmatic air-fluid collection is observed with pyogenic abscess or a superinfected amebic abscess . In addition to ultrasonography or liver CT, a liver scan usually accurately localizes the abscess. CT should routinely be enhanced with intravenous injection of contrast medium to demonstrate the relative hypovascularity of the abscess. Specific Imaging Tests Ultrasonography. Ultrasonography is the most useful screening test when the suspicion of hepatic abscess arises. The test is highly sensitive (85% to 95%), is more accurate than CT in imaging the biliary tree, and allows diagnostic or therapeutic drainage or biopsy at the time of performance. Limitations of ultrasonography arise in nonhomogeneous livers, in those situated high beneath the thoracic cage, and in extremely obese or uncooperative patients. Computed Tomography. CT is the most sensitive of the imaging procedures (95% to 100%) and allows diagnostic or therapeutic intervention to be performed. The appearance of an abscess on a CT scan is variable, and lesions may appear cystic or isodense, with solid metastatic lesions. A minority of hepatic lesions contain gas, making this criterion generally of little use but diagnostic if present. The phenomenon of an enhancing rim surrounding an abscess is apparent in a small percentage of cases. Radionuclide Scans. Technetium-99m sulfur colloid scanning has been useful in the diagnosis of abscesses for four decades. The labeled colloid is engulfed by hepatic Kupffer's cells, in which the technetium is reduced to a lower energy state, and the radiation emitted is measured by a photon-sensitive crystal. The ability of the test to demonstrate an abscess lies in the difference of Kupffer cell activity within and surrounding the abscess. The test is very sensitive but has the significant limitations of being unable to detect lesions smaller than 2 cm. as well as to discriminate between solid and cystic structures. The liver scan is an adequate screening test but is not useful in planning treatment strategies. Newer radionuclide scans with gallium and indium have been reported but add nothing to the standard technetium scan and are not as useful as ultrasonography or CT. Other Techniques. Hepatic arteriography has been used in the past in diagnosing pyogenic abscesses, but this invasive technique offers no benefit over CT scan with percutaneous aspiration for diagnosis. At present, magnetic resonance imaging offers no advantage over CT and cannot be used for percutaneous procedures. The diagnosis of the underlying source may be apparent from the history, physical examination, or initial laboratory tests. If ultrasonography and CT do not reveal the underlying source, barium studies, colonoscopy, endoscopic retrograde cholangiopancreatography, or other tests may be indicated. DIFFERENTIAL DIAGNOSIS Correct and expedient diagnosis of pyogenic versus amebic abscess is important because the treatments are radically different . The standard treatment of pyogenic abscess remains external drainage with an appropriate course of antibiotics. Management also includes consideration of the underlying cause of the abscess. Treatment of uncomplicated amebic abscess is primarily nonsurgical. When an abscess has been demonstrated, the distinction must be made between pyogenic and more unusual types. Echinococcus can usually be excluded by history. Serum antibody titer for E. histolytica and counterimmunoelectrophoresis are highly specific and of great benefit when positive. Major centers should have results available in 24 to 36 hours. Percutaneous aspiration may help in the identification of a bacterial organism. However, such aspiration is not usually helpful in the diagnosis of amebae, and in only 10% to 20% of cases can amebae be found on microscopic analysis of rectal mucosa secretions. If the latter results are positive, the finding still may be coincidental with a pyogenic abscess. If amebic serologic testing is not available or the results delayed, the best method of early distinction between pyogenic and amebic abscess is a trial of an amebicidal agent. Metronidazole is usually selected because it is also effective against many organisms causing pyogenic abscess. If the patient has not responded clinically in a 24- to 36-hour trial, pyogenic abscess should be the primary diagnosis. Clinical response is determined by relief of pain, fever, and leukocytosis. The radiologic and chemical features of amebic abscess are nearly indistinguishable from those of a solitary pyogenic abscess. Leukocytosis is characteristic, although a white blood cell count in excess of 20,000 per cu. mm. suggests secondary bacterial infection. Symptoms are also similar, except that rigors are rare in amebic abscess. MANAGEMENT Pyogenic Abscess Untreated pyogenic abscesses are 95% to 100% fatal. Death follows rupture, sepsis, or both. Spontaneous drainage is most often directly into the peritoneal or pleural cavity, usually causing septic shock and death. Rarely an abscess can resolve by spontaneous drainage externally or into the intestine. The likelihood of rupture is related to size and location. The larger the size, the more prone the abscess is to rupture, and left lateral segment lesions are more likely to rupture than right lobe lesions. Prognostic factors include the patient's age, multiplicity of abscesses, multiplicity of organisms, and the presence of associated malignant or other immunosuppressive disease. Survival from pyogenic abscess has improved in recent years with earlier diagnosis and treatment. Mortality was 80% in 1938 and over the past decade has decreased to less than 20%. Effective management of pyogenic abscess involves elimination of both the abscess and the underlying source. At the time of diagnosis of the abscess the underlying problem has usually been present for a variable period and the concern is usually focused on treatment of the abscess. The treatment of pyogenic abscess usually includes both intravenous antibiotics and effective drainage. A 1- or 2-day period of intravenous antibiotics may be reasonable before drainage. During this time, the patient's clinical response is closely observed and possible sources are evaluated. Effective drainage is accomplished by percutaneous or open surgical methods. Factors that should be considered in the selection of method include available expertise, the accessibility of the abscess, the number and size of the abscesses, and treatment of the underlying condition. The course of management must be individualized. For example, the combination of appendiceal and hepatic abscess is usually best treated by open drainage of both. However, percutaneous drainage may be useful as definitive therapy in many patients, in the preoperative period in a septic patient not adequately treated by antibiotics or in a patient for whom a search for a primary source continues. An example of an abscess particularly accessible to percutaneous drainage is a solitary one in the peripheral posterior aspect of the right lobe. In contrast, a large left lateral segment lesion may be more prone to spillage into the peritoneal cavity and the controlled exposure of laparotomy may be of benefit. Numerous small abscesses cannot usually be effectively drained and should be treated initially by antibiotics alone, although percutaneous aspiration for culture may aid in the choice of antibiotics. Drainage and antibiotics are unlikely to be curative in two exceptional situations: secondary infection of a hepatic malignancy and hepatic abscess associated with chronic granulomatous disease of childhood. In both instances the best treatment is hepatic resection if safely achievable. Interferon gamma has been reported to be effective in the treatment of chronic granulomatous disease of childhood. The drug has been given alone or after operation. Prophylactic interferon gamma (0.05 mg./sq. m.) three times weekly in these children may help prevent hepatic abscesses. Recent series have documented the ease, effectiveness, and safety of percutaneous drainage of most hepatic abscesses. Percutaneous drainage has the disadvantages of an increased likelihood of secondary procedures and the possibility of an underlying source remaining unknown. Some surgeons have reported a high incidence of sepsis in association with the procedures. Fortunately, repeated percutaneous drainage attempts are associated with little increase in mortality. Multiple abscesses remain difficult management problems by either percutaneous, open, or even a combination of the two approaches. The variability in patients precludes a good prospective study of percutaneous versus open surgical drainage. Most studies report a higher rate of complications and mortality with open drainage but include patients who required open drainage after percutaneous methods had failed. Any comparative analysis must closely consider the underlying conditions involved in the abscesses because these are changing (e.g., the increased incidence of abscess as a complication in immunosuppressed patients). Continuing studies are evaluating intravenous antibiotics as primary therapy for hepatic abscess. However, nondrainage therapy must presently be considered still experimental, considering the high risk of this treatment in many previous retrospective analyses. Adjunctive antibiotic therapy is critical to effective treatment of hepatic abscesses. Directed therapy may be based on results of Gram's stain or culture of diagnostic abscess aspirates. The most common isolates are gram-negative aerobes, colonic anaerobes, and microaerophilic streptococcal species. Anaerobes are rarely isolated singly and therefore necessitate broad coverage if discovered. An appropriate regimen might include an aminoglycoside; an antibiotic directed primarily against anaerobes, such as clindamycin or metronidazole; and a penicillin. This regimen is adjusted appropriately after definitive culture results become known. The appropriate length of antibiotic therapy is unknown, but recommendations include 3 to 12 weeks. The duration of drainage, of course, depends on the method and effectiveness of external drainage. The choice among several surgical approaches for open drainage of hepatic abscesses has been a controversial subject for many years. Three approaches are available: transpleural, extraperitoneal, and transperitoneal. Percutaneous drainage has eliminated much of this controversy. Most surgeons prefer the transperitoneal route if the abscess requires open surgical therapy. This approach allows inspection of the entire abdominal cavity for an underlying source as well as the best mobilization for appropriate drainage. Intraoperative ultrasound has added to the surgeon's ability to detect multiple hepatic or extrahepatic sites. The transpleural route is occasionally useful for high posterior lesions. The extraperitoneal route is usually through the twelfth rib (posterior) approach or an anterior retroperitoneal dissection. Recent reports also demonstrate three distinct advantages of laparoscopic drainage of pyogenic abscesses. These are the potential use of larger drains, avoidance of transthoracic drainage, and the search for a primary source of infection. Percutaneous drainage is accomplished by localization of the abscess with ultrasound or CT guidance . A Chiba needle is introduced through the safest anatomic route possible; and when the cavity is located, a trocar or an 18-gauge sheathed needle is passed with progressive dilatation of the tract using a guide wire and sheath. An 11- to 14-French pigtail catheter is subsequently left within the cavity, and it is irrigated with sterile saline and attached to gravity drainage, with the catheter being irrigated two or three times daily. The sterile pleural space is usually avoided in this technique. Amebic Abscess Except when there is rupture or secondary infection, amebicidal agents are the treatment of choice for hepatic amebiasis. The best drug is usually metronidazole or a related agent. Alternative drugs include emetine, dehydroemetine, and chloroquine. The usual dosage of metronidazole is 750 mg. orally three times a day for 7 to 14 days. If the patient is too ill to receive oral agents, intravenous administration is effective. For acute intestinal amebiasis, the recommended dosage is similar. The dosage for amebic hepatic abscess in children is 35 to 50 mg. per kg. every 24 hours in divided doses for 10 days. Emetine and dehydroemetine may cause cardiotoxicity but may be useful if metronidazole is not curative. If clinical symptoms do not resolve within 48 hours after treatment, an incorrect diagnosis or secondary bacterial infection should be suspected. Peritoneal aspiration or surgical drainage may then be considered. Surgical therapy also has a role in suspected rupture, erosion, or perforation of an adjacent viscus and extrahepatic problems such as colonic obstruction and toxic megacolon. Such colonic problems are extremely rare; and when they occur, they usually involve the right colon. Mortality from amebic liver abscess should be less than 5% in the absence of secondary bacterial infection. III. NEOPLASMS OF THE LIVER NEOPLASMS OF THE LIVER The development of hepatobiliary surgery as a distinct specialty reflects the increasing sophistication in the management of liver tumors. Health care economists have identified specialty care such as this as costly. However, the benefits of these operations are great, and the costs are minimized by early detection of liver neoplasms and appropriate and skillful treatment. During the past decade surgeons have encountered more liver tumors. This increase is due to four principal factors: (1) an apparent increase in incidence of tumors; (2) improved detection; (3) continued enthusiasm about the success of surgical treatment; and (4) combined, aggressive approaches to reduce tumor volume even in apparently incurable situations. In addition, over the past 30 years several new categories of tumors have been recognized. New etiologic factors have been identified, such as the hepatitis B and C viruses, hemochromatosis, exposure to vinyl chloride, oral contraceptive use, Thorotrast injection, liver adenoma, and chronic liver disease of almost any etiology. Other factors have been implicated, such as agent orange (dioxin), and there is renewed interest in other types of environmental induction. In contrast to other oncologic diseases, primary liver cancer seems much more a disease of acquisition than of genetic predisposition. The challenges of cost effectiveness in this dynamic specialty are considerable. For example, computed tomographic (CT) portography, magnetic resonance imaging (MRI), and positron emission tomography have greatly increased the sensitivity and specificity of preoperative imaging. These tests are also, to a certain degree, complementary. Therefore, thoughtful algorithms concerning their use are important, as is early operation in appropriate cases. It is also interesting to reflect over the past 5 or so years and note the development of the surgeon as a diagnostician in addition to a therapist. This development closely parallels the increased use of laparoscopy. Laparoscopic cryoablation of liver tumors may be a culmination of modern technology with respect to both diagnosis and therapy. THE SURGEON'S DEVELOPING ROLE The traditional role of the hepatic surgeon has been to safely control the liver's blood supply during surgery. The earliest hepatic surgery was performed almost exclusively for trauma. The earliest resection for tumor was by Langenbuch in 1888. In 1889, Keen reviewed the liver resections performed to that time. In 1911, Wendel performed the first successful major hepatic resection using selective hilar ligation of the vessels. With improved understanding of the anatomy and physiology of the liver has come increased confidence in surgical treatment, a significant increase in the number of resections performed, a wide expansion of the indications for resection, and a reduction in the mortality for elective hepatic resection in selected major liver centers to less than 1%. Much of modern medicine seems to be focusing on cost issues and the streamlining of health care delivery. In deference to the modern focus on cost issues, this chapter is reorganized slightly to conform to recent algorithms. The surgeon approaches hepatobiliary disease in the modern era in terms of two basic categories: operable and inoperable. Operable can be variably defined to include or exclude intervention such as endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography (PTC), or the new transjugular intrahepatic portosystemic shunt (TIPS) procedure. Treatment of many problems is changing. For example, operable diseases of 10 to 20 years ago are now treated by ERCP, and nonoperable diseases from that era are now treated by transplantation. The current classifications (Table 33–6 Table 33–6) are meant to allow a general approach to the management of the various hepatobiliary problems, not definitive categorization. Operable liver disease in its new classification means hepatobiliary disease treated by open or laparoscopic operation. The three main categories are hepatic resection, transplantation, and other surgical options. Considerable overlap occurs, of course, among the various options. In this discussion the focus is on resectable liver disease, specifically neoplastic disease, cystic and parasitic disease, and several other specific lesions. This subject is discussed elsewhere in this text in the discussions of liver transplantation and portal hypertension. PRIMARY MALIGNANT TUMORS Familiarity with the types and relative incidences of primary or secondary liver tumors is important for both the surgeon and primary care physician. Take, for example, the workup of a young woman with right upper quadrant pain. One could perform ultrasonography as the initial diagnostic test and then order a battery of more expensive imaging tests, blood chemistries, hepatology or general surgery consultations, ERCP, PTC, and biopsies. An alternative scenario might be early laparoscopy with definitive diagnosis and surgical treatment at the same operative sitting. Hepatocellular Carcinoma Hepatocellular carcinoma (HCC) is the most prevalent malignant disease in the world, killing up to 1.25 million persons annually. It constitutes 90% to 95% of primary liver cancers. HCC is the most common primary malignant liver tumor in adults. Hepatoblastoma is the most common primary hepatic cancer of young children. The variability and prevalence of HCC remain dramatic. This malignancy has nearly doubled in the United States over the past 20 years. Hepatoma is much more common in sub-Saharan Africa, Southeast Asia, Japan, the Pacific islands, Greece, and Italy than it is in North and Central America, Great Britain, most parts of Europe, the Middle East, the former Soviet Union, and Australia. In Africa, the incidence of HCC varies from 13.5 per 100,000 annually in Swaziland to 143.8 per 100,000 individuals in Mozambique. In contrast, North Africa has an incidence that varies from 0.8 per 100,000 to 9.0 per 100,000 in Sudan. Five different studies demonstrate HCC to have doubled over the past 30 years in the United States. HCC occurs four to nine times more frequently in males than in females, except in the group without pre-existent liver disease, in whom the ratio is 1:1. Asians in the United States are approximately eight times at risk for developing the tumor compared with white populations. HCC makes up a relatively small proportion of the total primary cancers diagnosed on a yearly basis in the United States. It has an annual incidence in the United States of 1 to 7 per 100,000 population and is the twenty-second most common type of cancer. Studies of histocompatibility antigens in southern African blacks do not support a genetic basis for the increased susceptibility to HCC. The higher risk of men in certain populations probably reflects a greater exposure to environmental carcinogens. The differences include not only a higher hepatitis B carrier rate in males but also dietary carcinogens and varying rates of metabolism within the sexes. Chinese patients with HCC are usually considerably older than their black counterparts. There are rural-born blacks in South Africa who migrate as young adults to cities and are at lower risk from the disease. HCC occurs about two decades later in blacks who remain in the rural setting. The carcinogen aflatoxin is increasingly implicated. In populations with low incidences of HCC, the elderly are the predominant group affected. In the Far East, HCC develops predominantly in the fifth and sixth decades. In sub-Saharan Africa, the tumor develops at a younger age, with a peak incidence in the third through fifth decades. In high-incidence locations, the tumor more often occurs in patients treated for cirrhosis for a long period of time. Etiology. Chronic liver disease of any cause probably plays an important role in development of HCC in any part of the world. Epidemiologic and laboratory studies have firmly established a strong and specific association between hepatitis B virus (HBV) and HCC. Other well-documented risk factors include alcoholic cirrhosis, blood group B, hepatic adenoma, repeated ingestion of aflatoxin, other types of cirrhosis or chronic active hepatitis, and persistent hepatitis C viral infection. Implicated risk factors for HCC include other various mycotoxins, plant alkaloids, oral contraceptives, androgens, vinyl chloride, Thorotrast, parasites, porphyria, organochloride pesticides, membranous obstruction of the inferior vena cava, hemochromatosis, alpha 1-antitrypsin deficiency, and even cigarette smoking. The relative risk of each factor also appears to vary by geography, although the reason for this difficulty may be both incomplete reporting and the presence of more than one major risk factor. Hepatitis B Virus. The evidence for the causal relationship between chronic infection with HBV and the disease is compelling and has accumulated over the past two decades. As many as 98% of indigenous persons are infected with the virus at some time in their lives, and approximately 10% are persistently infected. Studies from both Asia and Africa suggest that HBV is present in as many as 70% to 80% of patients with HCC. In 97 consecutive autopsies of patients with HCC in Los Angeles, 21% had HBV infection. In addition, 40% of 55 patients dying of HBV cirrhosis had HCC at autopsy. One third of the patients with HCC studied at autopsy had a cryptogenic cirrhosis. Whether HCC occurs as a consequence of chronic HBV infection or as a result of chronic liver disease is not certain. Since HBV vaccine has been available for only a short period of time, it is not known if this will have a significant impact on the incidence of the disease in endemic areas. HBV does not contain an oncogene, but insertional mutagenesis is a potential mechanism. Overexpression of c-myc, c-phos, and c-erb-A oncogenes has been demonstrated in tumors from African blacks, but the relationship may be coincidental. A number of other studies in individual patients or cell lines has suggested lost tumor suppressor genes. The relative risk of developing HCC among HBV carriers is 9.7. Observations that strongly implicate HBV are as follows: (1) the HBV carrier state exists in the same geographic distribution as HCC; (2) HCC patients have a 100-fold greater sensitivity to hepatitis B surface antigen (HBsAg) than do population-matched controls; (3) HBV infections precede the development of HCC; (4) in a 5-year study, HBsAg-positive patients had a 1000-fold increased risk of developing HCC; (5) progression from chronic active HBV to cirrhosis and subsequently HCC is well documented; and (6) HBV DNA resides in the HCC cell genome. Hepatitis C. In contrast to HBV infection, most of the information showing an association between chronic hepatitis C virus (HCV) infection and HCC has been accumulated in Western civilizations, where there is relatively little overlap with HBV. HBV and HCV do act as independent risk factors, plus the two viruses may have a synergistic effect. HCV is a single-stranded RNA that does not become integrated host to DNA. Therefore, it is not certain whether the virus is directly carcinogenic or its marked propensity to cause chronic necroinflammatory disease is the real culprit. Interaction of either HBV or HCV with the p53 protein might also provide a growth advantage to cells harboring a mutation. Studies in Japan have also implicated HCV as the predominant cause of cirrhosis and HCC. HCV infection is present in 51% of the patients with HCC in Japan, as opposed to 26% with HBV infection. Other Risk Factors. Alcoholic cirrhosis is certainly a major predisposing factor for HCC in the United States; 8% to 10% of patients dying of alcoholic cirrhosis have HCC. In one study, 55% of patients with alcoholic cirrhosis who have stopped drinking had HCC at autopsy, leading to the suggestion that abstinence from alcohol use allows the alcoholic patient to live long enough to develop a tumor. Aflatoxins are important carcinogens in experimental animals. They are products of the fungus Aspergillus flavus, which is found in wheat, soybeans, corn, rice, oats, bread, milk, cheese, and peanuts. The United States Food and Drug Administration limits the amount of aflatoxins allowed in peanut butter to 20 parts per billion. Ingestion of raw materials is another likely reason for the geographic variability of HCC. In Taiwan, the risk may be regulated through a guanine to thymine mutation in the p53 suppressor gene. The exact risk of HCC developing with the use of oral contraceptives is not clear, although a number of carcinomas have been reported arising within benign adenomas in oral contraceptive users. A type of carcinoma termed fibrolamellar carcinoma characteristically develops in persons younger than the age of 35, and it is possible that some of these tumors are also linked etiologically to oral contraceptives. A number of cases of hepatoma have been reported in males after administration of antigenic or other anabolic steroids for the treatment of aplastic anemia, although it is also possible that multiple transfusions contributed to anemia in these patients. There does appear to be a greater hormone responsiveness in these androgen-related HCCs. Most of the research relating to coexistence of cirrhosis in HCC has focused on the high turnover rates of those cells. Interestingly, primary biliary cirrhosis did not appear to be associated with HCC. On the other hand, the reported association of HCC with hemochromatosis-related cirrhosis is between 10% and 30%. Symptoms and Signs. The most common symptoms of HCC relate to its late discovery. They include weakness, malaise, anorexia, upper abdominal pain, and weight loss. In one series in the United States, an abdominal mass was the main complaint in 19 of 140 of patients (14%) with primary hepatic malignant tumors and jaundice was present in 34 patients (24%). Obstructive jaundice is the initial problem in 1% to 10% of patients. The biliary obstruction is caused by necrotic tumor embolism or extrinsic compression of the biliary system. Approximately two thirds of the patients are hospitalized with an obvious cancer in the liver with symptoms or signs including abdominal pain and tenderness, dyspnea, asthenia, weight loss, hepatomegaly, jaundice, ascites, peripheral edema, or evidence of portal hypertension. Approximately 5% of the patients present with a manifestation of metastatic lesions, of which pulmonary metastases are the most common. A minority of patients initially experience an acute abdominal event, such as rupture of the tumor or hemorrhage, or fever of unknown origin. More often, the tumor represents an occult process. In endemic areas, HCC is the most common cause of nontraumatic acute hemoperitoneum in males. In general, the duration of symptoms of patients with HCC is often surprisingly short. In one series, over 75% of patients had symptoms of less than 6 weeks' duration. Physical findings depend on the stage of the disease. Hepatomegaly is the most common sign found in patients. Interestingly, an arterial bruit can be heard in 15% to 20% of patients, which may be diagnostically valuable. Two thirds of the patients admitted with an obvious cancer exhibit many signs of liver disease (e.g., abdominal pain and tenderness, dyspnea, asthenia, hepatomegaly, splenomegaly, jaundice, ascites, peripheral edema, weight loss, spider angiomas, or evidence of portal hypertension). The few patients who initially have an acute abdominal event representing rupture or hemorrhage into the liver (6.9%) or peritoneal cavity generally have a poor prognosis. Ninety percent of the patients have metastatic disease at initial presentation. Many paraneoplastic manifestations are associated with HCC. The most common of these manifestations is probably hypoglycemia. Other manifestations include serum protein abnormalities of alpha-fetoprotein, globulins, haptoglobin, ceruloplasmin, alpha 1antitrypsin, choriogonadotropins and choriosomatotropins, alkaline phosphatase, and isoferritins. Associated hematologic abnormalities include erythrocytosis, hemolysis, plasmacytosis, and dysfibrinogenemias. Other abnormalities include hypercholesterolemia, hypertriglyceridemia, porphyria, cystathioninuria, ethanolaminuria, pseudohyperparathyroidism, sexual changes, hypertrophic pulmonary osteoarthropathy (hepatopulmonary syndrome), increased thyroxine-binding globulin, and carcinoid syndrome. Increased carcinoembryonic antigen (CEA) occurs in 8% of cases. Other hormonal syndromes include hypertension secondary to the overproduction of angiotensin, a sensorimotor polyneuropathy that affects all four limbs, and hyperthyroidism caused by increased circulatory thyroid-stimulating hormone, thyroxine, triiodothyronine, and free triiodothyronine. Diagnosis. The oncofetoprotein alpha-fetoprotein (AFP) deserves special mention because of its diagnostic value. This protein has a molecular weight of 64,000 to 74,000 daltons. It is present in large quantities during fetal development but decreases rapidly after birth and thereafter remains at the normal adult level of 10 ng. per ml. or less. When a relatively insensitive immunodiffusion method is used, 28% to 87% of patients with HCC are shown to have significant elevations of AFP in their sera. Radioimmunoassay for AFP increased positivity for tumor detection in Chinese patients to 69% to 93%. In the United States, 75% of patients with HCC arising in association with HBV cirrhosis had AFP levels above 400 ng. per ml. Sixty-five percent of patients with HCC secondary to alcoholic cirrhosis had positive results, whereas only 33% of patients with carcinoma arising in a noncirrhotic liver had positive assays. In one study, 14 of 16 patients who underwent laparotomy for a positive AFP level found on screening tests had resectable cancers. AFP may return to normal after successful surgical resection and is a useful level to follow. Mild elevations of AFP may be found in acute viral hepatitis, chronic liver disease, and some cases of metastatic cancer. Higher levels may be found in adult patients with fulminant type HBV infection. Markedly elevated levels may also be found in patients with teratocarcinomas, yolk sac tumors, and, rarely, hepatic metastatic carcinomas from the stomach or pancreas. Recently the value of real-time ultrasonography has been realized in both Japan and Taiwan as a screening test for HCC in high-risk populations. One study in Japan disclosed that ultrasonography detected 72.5% of tumors that developed in a group of patients with chronic liver disease undergoing routine follow-up evaluation. In another study, ultrasonography detected 92% of HCCs less than 5 cm. in diameter, compared with scintigraphy, which demonstrated only 50%. Arteriography confirmed the majority of lesions. It was also learned that ultrasonography performed at 4- to 5-month intervals theoretically detects all tumors less than 3 cm. in diameter. The time interval was determined by calculation of estimated tumor growth rate. In one study, 4.6 months ensued for a rapidly growing 1-cm. HCC to reach the size of 3 cm. In one prospective study of 115 patients with cirrhosis in Japan, HCC developed in 12 (10.4%): the tumor developed in 7 of 30 (23%) HBsAg-positive patients and 5 of 85 (6%) seronegative patients. A more recent development is the use of intraoperative ultrasonography for the detection of occult tumors. Several reports now document the usefulness of this technique in combination with preoperative AFP determinations. In the United States, three fourths of patients with an HCC greater than 5 cm. in diameter have AFPs greater than 100 mg. per liter (normal, 0 to 20 mg/L). A level greater than 4 mg. per liter is generally diagnostic for HCC. Acute hepatitis usually produces only transient rises in AFP, whereas chronic hepatitis generally produces a low level AFP elevation, which correlates with the degree of transaminase elevations. Therefore, minor elevations in the AFP level in such patients prompt re-evaluation in 3 months. From 10% to 15% of liver tumors do not produce AFP; therefore, ultrasonography is a better screening study in general for HCC. A National Institutes of Health Consensus Panel recommends the use of both ultrasonography and AFP to detect HCC in high-risk populations. A number of radiologic investigations may be helpful in the diagnosis of HCC (Fig. 33–20 Fig. 33–20). Plain radiographs are nonspecific and may show an enlarged liver, elevated hemidiaphragm, and, rarely, calcification of the tumor. Ultrasonography is generally noninvasive and expensive. Radionuclide scans are relatively sensitive but have more false-positive results than ultrasonography or CT. Lesions less than 1 to 2 cm. in diameter are often not detected by ultrasonography or radionuclide scans. CT, with and without enhancement with intravenously injected contrast material, has emerged as the procedure of choice in most cases to define the HCC. CT may detect lesions as small as 1 cm. in diameter, and it may also differentiate among fatty, cystic, and solid lesions. However, MRI has probably overtaken conventional CT with respect to sensitivity and characterization of the tumors. Hepatic arteriography is occasionally helpful in determining the extent of the disease and, in particular, portal or arterial involvement. It can also predict the usefulness of direct arterial infusion or chemoembolization for HCC, which may be either hypervascular or hypovascular. A preoperative arteriogram may also be helpful in determining anatomic variability. A distinct disadvantage of arteriography is the possibility of thrombosing an artery to the remaining lobe when major hepatic resection is anticipated. Splenoportography may demonstrate the intrahepatic spread of tumor and invasion of the portal vein, but the procedure is not widely used because invasion may also be seen during the venous stage of arteriography. Percutaneous or retrograde cholangiography may be helpful in selected patients. Laparoscopy is emerging as a procedure of choice in the diagnosis of HCC or other suspected liver tumors. Five separate studies demonstrate its usefulness before anticipated resection, particularly in the detection of occult metastases not demonstrated by other modalities. Laparoscopy also offers an opportunity for limited treatment. An unpublished Duke University series included 22 patients who underwent laparoscopic cryoablation, alcohol injection, minor resection, or a combination of therapies. Percutaneous needle biopsy or fine-needle aspiration for cytodiagnosis generally adds little to the evaluation of potentially resectable, indeterminant liver masses, plus needle biopsy or aspiration has some hazard for hypervascular masses. These techniques are reported to diagnose two thirds of HCCs, and an indeterminant or negative biopsy aspirate adds little to the decisionmaking process, except perhaps to establish cirrhosis. Pathology. Various classifications confuse the understanding of the pathology of HCC. The traditional classification of Eggel divides the tumors into massive, nodular, or diffuse. The more recent classification of Nakashima has four groups: infiltrative, expansive, mixed infiltrative and expansive, and diffuse. The latter classification also recognizes two special gross types of HCC: small (less than 2 cm. in diameter) and pedunculated. The classifications are further divided by histopathology. The most common histologic pattern is trabecular, which encompasses the pseudoglandular, pseudofollicular, and mixed trabecular-acinar types. Other histologic adjectives often used with HCC include pseudoglandular, solid, compact, scirrous, clear cell (replacing), giant cell, pseudocapsular, and sarcomatous. More important than these classifications are the recognition of distinctive variants of HCC and the presence or absence of cirrhosis. The gross pathologic appearance of HCC varies considerably, depending on the presence or absence of pre-existing cirrhosis. In the cirrhotic liver, it is most often multinodular, whereas in the noncirrhotic liver, it is usually a single mass. The tumor probably begins in an otherwise normal liver as a fairly homogeneous mass and then develops satellite lesions. Most satellite lesions occur within the same segment. The more diffuse multifocal disease in the cirrhotic liver is probably a function of the end-stage disease of the liver. Distinctive HCC Variants Fibrolamellar HCC. During the past two decades, the fibrolamellar variant of HCC has emerged as a distinct clinical and pathologic entity. The tumor occurs primarily in young patients with noncirrhotic livers and has a more favorable prognosis than does standard HCC. The distinguishing histologic features are sheets of well-differentiated hepatocytes sandwiched between lamellae of collagen and fibroblasts neoplasm accounts for only 1% to 2% of all HCCs, but as many as 40% of such tumors in patients younger than 35 years old. A female sex predilection is debated. Two thirds of such tumors occur in the left lobeCharacteristic gross features include a sharply circumscribed solitary mass with scalloped borders, often with adjacent small satellite nodules, fibrous septa within the tumor that mimic the central scars of focal nodular hyperplasia, and a distinct vascular supply. The resemblance of fibrolamellar carcinoma to focal nodular hyperplasia suggests malignant transformation, but this remains unlikely. The cause of the tumor remains uncertain. The location and distribution of metastases are similar to those in HCC, except in association with a more prolonged course. Fibrolamellar carcinoma usually remains well localized at the time of diagnosis, yielding a 50% to 75% resectability rate. Mean survival from the time of diagnosis is 32 to 68 months. An aggressive operative approach to this disease is obviously warranted. Childhood HCC. The underlying hepatic disorders that lead to HCC in childhood distinguish this category of HCC. Those disorders include biliary atresia, glycogen storage disease Type I, hereditary tyrosinemia, hyperalimentation, and HBV infection. In most cases, the gross features of childhood HCC include multifocality and bilaterality. The histologic features may resemble the underlying problem such as glycogen storage disease. It is not clear whether these patients have a different prognosis. Spindle Cell HCC and Carcinosarcoma. An increasing number of tumors show features of both HCC and spindle cell carcinoma. For this reason, the term carcinosarcoma is usually reserved for similar tumors but with the sarcoma being a non–spindle cell variety. Pedunculated HCCs represent an unusually large number of these tumors. Although metastases may be more common in these tumors, these patients may actually live longer than those with the more common HCC. Clear Cell Carcinoma. This tumor represents a significant problem distinguishing it from metastatic renal cell carcinoma. In fact, the two tumors have been found to coexist in the same patients. The Hong Kong experience suggests that this cell type has a better prognosis, but all patients in that series had a generally short survival. A better prognosis has not been reproduced in other series. Giant Cell Carcinoma. This tumor refers to carcinomas in which more than 50% of the primary tumor is composed of either multinucleated or pleomorphic large cells. The exact cell of origin is not apparent but certainly may be hepatocellular. These tumors apparently have a similar prognosis to standard HCCs. Combined HCC (Hepatocellular-Cholangiocellular). Ductal transformation of HCCs actually occurs in 5% to 10% of tumors. The term mixed hepatic tumor refers to a distinct tumor type in which both hepatocellular and mesenchymal components are present. A number of patients have also been reported with both entities as separate tumors. Histologic evidence suggests that these tumors are similar to the spindle cell carcinoma/HCC variant, but the prognosis with this tumor is not clearly different from that of standard HCC. Other Primary Malignant Tumors Epithelial Tumors Hepatoblastoma. Hepatoblastoma is a malignant tumor of embryonic or fetal hepatocytes that often has mesenchymal elements and occurs in children younger than 3 years of age. The tumors are generally divided into epithelial or mixed epithelial and mesenchymal types, depending on the number of mesenchymal cells seen. Other terms employed for some tumors are teratoid and anaplastic. These tumors are responsible for 50% of all primary hepatic tumors requiring surgery in a 10-year survey of pediatric surgeons in the United States. These tumors are about one tenth as frequent as Wilms' tumors. There is a white male predominance, and metabolic effects include osteopenia, hypoglycemia, isosexual precocity, and hemihypertrophy. Possible striking effects include marked elevations of beta-human chorionic gonadotropin, thrombocytosis, serum alpha-fetoprotein elevation, cystathioninuria, virilization, and radiographic calcification. Cirrhosis is usually absent. The tumor is often solitary but multinodular with a variegated appearance. Over 50% long-term survival in patients who undergo resection of the tumor is expected. Recurrence can occur in up to half the patients. Several recent studies suggest that prognosis is predicted by histologic grading and ploidy analysis. Large-scale studies of therapy for this tumor have not been reported. Bile Duct Cancer (Cholangiocarcinoma). Several authors suggest using the term bile duct cancer for central lesions and cholangiocarcinoma for tumors in more peripheral ducts. The terms are nonetheless interchanged in the literature. Bile duct cancers represent 5% to 20% of primary carcinoma of the liver. They may occur within the liver parenchyma and small ducts or ductules or arise from major hepatic ducts outside the liver substance. The intrahepatic type of cholangiocarcinoma is associated with chronic cholestasis, cirrhosis, hemochromatosis, and congenital cystic disease of the liver. Clonorchis sinensis infestation is associated with more than 90% of cholangiocarcinomas in Hong Kong. However, one third of patients without bile duct carcinoma also have infestation with this fluke. Extrahepatic bile duct cancers arise anywhere along the hepatic ducts or bile duct and represent 10% of primary hepatic malignancies. There is a 3:2 male-to-female predominance. Pruritus, vague abdominal pain, mild cholangitis, and jaundice are the usual initial symptoms. Physical examination may reveal slight hepatomegaly or jaundice but usually no other findings except a large gallbladder when the lesion is distal. Both intrahepatic and extrahepatic bile duct cancers have been associated with ulcerative colitis and may also be confused with sclerosing cholangitis. There is a clear association with biliary cystic disease as well as with gallstones. On pathologic examination the lesion is generally a markedly sclerosing adenocarcinoma. This tumor may extend into the parenchyma along the biliary ducts. Twenty per cent to 25% of the tumors are found to be resectable even when the lesion involves the bifurcation of the hepatic ducts (Klatskin tumors). The treatment of choice probably remains surgical resection, which provides longest survival. The average survival in one series after surgical resection was approximately 2 years. There is a 15% to 20% 5-year survival rate after resection. Most cures occur after resection of distal-third lesions; cure is unusual for proximal bile duct cancers. If the tumor cannot be resected, a bypass or intubation procedure may provide excellent palliation. Before these palliative procedures were used, survival was usually only several months. With more aggressive palliation, average survival has been extended to 1 or 2 years. Adjunctive iridium seed implantation through a biliary drainage tube is often employed, with or without external beam iridium, but whether this leads to significant improvement in survival remains unclear. Percutaneous or endoscopic biliary drainage is offered as a primary treatment for poor-risk surgical patients or those with obviously unresectable tumors. The long-term results of percutaneous or endoscopic drainage compared with open dilation and intubation have not been fully evaluated. Intrahepatic ductal enteric anastomoses, such as the Longmire or falciform ligament approaches, have had mixed success in the treatment of unresectable cholangiocarcinoma. One might expect that patients with bile duct cancer would be a particularly favorable group for hepatic transplantation because of the slow-growing and localized nature of the tumor. However, results of transplantation have generally been disappointing, primarily because of tumor recurrence. This has, in part, been the stimulus for cluster operations being performed by several transplant groups in which most of the upper abdominal viscera are transplanted en bloc. Results of this aggressive surgical procedure are disappointing. At present, chemotherapy is considered an ineffective primary treatment modality. Other primary predisposing factors for cholangiocarcinoma are primary sclerosing cholangitis, inflammatory bowel disease, intrahepatic calculi, and possibly certain environmental toxins. The Mayo Clinic series of primary sclerosing cholangitis patients suggested a 17% incidence of carcinoma development during a period of observation before transplantation. A review from Johns Hopkins Hospital suggests that early resection of the extrahepatic ductal system for primary sclerosing cholangitis reduces the incidence of carcinoma development. Hepatic Cystadenocarcinoma. Most hepatobiliary cystadenocarcinomas probably arise from hepatobiliary cystadenomas. A predisposing condition (i.e., pre-existent cyst) has been traced to about half of those tumors in one series. However, in general, the prognosis of patients with these tumors is poor in contrast to that of patients with pancreatic cystadenocarcinoma. The poor prognosis may be related to the relatively low number of resections performed for this disease. Resection does offer a chance for cure. Squamous Cell Carcinoma. The primary consideration with this disease is the possibility of a metastatic lesion. Primary squamous cell carcinoma of the liver has occurred with longstanding biliary or hepatic cystic disease and has been cured by resection. However, the tumor is rare. Primary Malignant Mesenchymal Tumors. Primary hepatic sarcomas have received much attention because of their association with vinyl chloride or Thorotrast. Angiosarcoma is the principal tumor associated with this agent. It usually occurs as multiple nodules of variable size that rapidly disseminate intravascularly. No single incidence of cure for angiosarcoma has been reported. The longest reported survivor (5 years) received radiation therapy. Other tumors such as leiomyosarcoma, fibrosarcoma, rhabdomyosarcoma, and mesenchymal sarcoma also rarely appear in the liver. Undifferentiated sarcoma is also generally associated with an extremely poor prognosis, although the authors had a patient who had undergone tumor resection who was a 5-year survivor. Epithelioid hemangioendotheliomas are considered malignant because of their characteristically diffuse involvement within the liver and ability to metastasize. Their clinical presentation and course are extremely variable, with most patients dying of liver failure 1 to 10 years after diagnosis. Other malignant tumors reported rarely as primary lesions in the liver include malignant fibrous histiocytoma, familial erythrophagocytic lymphohistiocytosis, lymphoma, teratoma, yolk sac tumor, schwannoma, osteosarcoma, and carcinoid. Treatment of Primary Malignant Liver Tumors Two common misconceptions about HCC are that it grows rapidly and that it is universally fatal . HCC is actually slower growing, in general, than other neoplasms, such as colon cancer and bronchogenic carcinoma. Resection has often resulted in cure, particularly in the absence of cirrhosis. Most carcinomas resected in the presence of underlying cirrhosis or chronic hepatitis recur in the liver, although this may not happen for several years. Whether the recurrences are actually new lesions arising in the presence of cirrhosis is not known. When one considers all persons within an endemic population such as Africa, the overall survival of patients with untreated HCC is only 3 to 4 months after symptoms appear. In the United States, the course seems more benign. Average survival after resection is reported to be approximately 3 years. Five-year survival rates after resection (including liver transplantation) in several large series varied from 11% to 46%. At present, resection is the only therapy that substantially prolongs survival. Exploration is indicated unless there is an obviously unresectable tumor or distant metastases or end-stage cirrhosis. Operative mortality from major hepatic resection has decreased from nearly 20% before 1950 to approximately 1% currently. The mortality still relates primarily to postoperative liver failure from accompanying cirrhosis. Prognostic indices have developed to calculate the risk of hepatectomy in HCC and cirrhosis. Most are based on the modifications of Child's classification, percentage of remaining liver tissue, and patient age. In cirrhosis, wedge resection is as effective as more radical procedures in many patients. In addition to detection of occult lesions, intraoperative ultrasonography helps to determine the extent of resection and identification of the precise segment of liver to be removed, which can be done after injection of stain into the portal vein to that segment. The impact of early discovery and treatment of subclinical HCC is best depicted in studies from Shanghai. The overall 5-year survival from that disease increased dramatically from 1.7% in the period from 1950 to 1966 to 7.1% in the period from 1967 to 1975 and 19.5% in the period from 1976 to 1984. This dramatic increase was clearly the result of improved detection and resective treatment of subclinical HCC. No 5-year survivor was reported in the absence of resection. Most patients underwent limited resection, which usually consisted of a 1- to 2-cm. margin around the tumor. In the 1980s, liver transplantation was performed only for otherwise unresectable tumors, with only a 20% 2-year survival. In contrast, there was an 80% to 90% cure rate, with coincidental cancers found at pathologic examinations of the cirrhosis after transplantation. Fibrolamellar HCC had a better prognosis with transplantation, that is, 30% to 40% 1-year tumor-free survival, compared with 10% survival of patients with nonfibrolamellar tumors. Most deaths after transplantation for tumor are attributable to tumor recurrence. Nonprospective studies suggest a doubling of the chance for survival with transplantation compared with resection, when resection is an option. This outcome is most evident in patients with cirrhosis and with solitary lesions less than 5 cm in diameter. These data may also support an aggressive, combined approach to HCC, to include resection or transplantation plus another modality such as chemotherapy, chemoembolization, or cryoablation. Because of the shortage of donor livers, some authorities argue that transplantation for HCC, with its best long-term survival of only 20% to 30%, is not acceptable. This argument has led to proposals in certain donor regions to stratify the donor pool according to disease, with the less favorable organ going to the patient with carcinoma. Cryoablation. Cryoablation has emerged as a potentially useful surgical technique in the treatment of HCC and certain other malignancies. The technique is effective in local kill of tumor, particularly after a double-freeze technique. The overall operative morbidity and mortality of cryoablation may be less compared with resection. Therefore the number of nodules that can be treated may increase or the margin of resection may widen. Preliminary analyses from Boston, Pittsburgh, and New Haven in the United States and from Australia all suggest a slight prolongation of survival for patients with malignancies that were otherwise unresectable. Long-term data on cryoablation of primary or secondary liver malignancies are not available. Nonetheless, the technique raises a number of interesting possibilities in terms of combined therapy. Nonsurgical Treatment. Nonsurgical treatment of HCC includes hepatic artery ligation, arterial embolization, intra-arterial chemotherapy, targeting irradiation or chemotherapy, direct tumor injection, or combination of methods. Iodine-121 antiferritin therapy combined with external radiation and/or chemotherapy has not achieved satisfactory results. Mean survivals were 5 and 10 months in AFP-negative and AFP-positive patients, respectively, in one carefully designed study. Interestingly, arterial embolization has yielded the best results in unresected patients. However, the improvement in survival is still less than 6 months by that approach. In some studies investigators have suggested improved survival with the combination of an external-beam linear accelerator and direct percutaneous ultrasound-guided alcohol injection. Because HBV infection is now unequivocally linked to development of HCC, it would appear that HBV vaccine would reduce the incidence of this disease in endemic populations. However, economic factors have thus far prohibited wide distribution of this vaccine in endemic parts of the world. The most consistent response rates (~25%) with respect to systemic chemotherapy have been reported with doxorubicin (Adriamycin). The best results have occurred in black South African patients. METASTATIC TUMORS Metastatic cancer comprises the largest group of malignant tumors in the liver. Most metastatic lesions likely arise in the liver as a result of primary shedding into the vascular system. According to a large autopsy study in the United States, bronchogenic carcinoma is the most common primary lesion causing hepatic metastases . Next in order of frequency are primary tumors in the prostate, colon, breast, pancreas, stomach, kidney, and cervix. The liver is involved in 60% to 66% of small cell carcinomas of the lung, 40% of lung adenocarcinomas, and 22% of squamous cell carcinomas. Some reports indicate that over 40% of patients with breast cancer develop liver metastases before death. In total, about 40% of patients dying with a solid tumor develop liver metastases. The tumor that most concerns liver surgeons in the United States is metastatic colorectal cancer. At least 20,000 patients with colorectal cancer develop hepatic metastases annually. Liver metastases are detected in 30% of these patients at the time of primary anastomosis. Between 10% and 20% of patients discovered with liver metastases from colorectal cancer have potentially resectable disease. Pathogenesis Primary tumors that drain into the portal system contribute seven times as many hepatic metastases as tumors arising outside the portal drainage system. Only 2% to 3% of patients with hepatic metastases have no detectable extrahepatic disease. The factors governing the distribution of hepatic metastases within the liver are not well understood. Unanswered questions include (1) why some patients have only one or several tumors confined to one lobe and others exhibit multiple tumors throughout the liver; (2) when hematogenous spread occurs; and (3) what other factors, such as age or sex, influence the natural history of hepatic metastases. Some factors governing number in distribution of hepatic metastases undoubtedly include the number and viability of tumor emboli, hemodynamic factors related to distribution of liver blood flow, receptiveness of the liver tissue to implantation and growth, and the tumor's inherent aggressiveness. Detection The most common symptoms of hepatic metastatic disease are pain, ascites, jaundice, palpable mass, weight loss, anorexia, fever, and vague gastrointestinal complaints. Most of these symptoms generally indicate advanced disease. Therefore, one cannot rely on development of symptoms for early detection of metastases to the liver. A correlative observation is that most resectable lesions are found by early laboratory detection before symptoms or signs develop. For this reason, close follow-up of patients with colorectal cancer is required. Liver enzyme and CEA determinations in combination with appropriately timed imaging studies are recommended in the routine follow-up of patients with colorectal cancer. CEA is a 200,000-dalton glycoprotein secreted into the glycocalyceal surface of intestinal cells. The human digestive organs elaborate CEA between the second and sixth months in utero. Human colorectal cancer often secretes sufficient amounts of CEA to permit its use as a tumor marker. An elevated CEA level was formerly believed to be specific for colorectal malignancy. Unfortunately, this is not the case, because increased CEA concentrations occur in other malignancies and in benign conditions such as alcoholic cirrhosis, pancreatitis, inflammatory bowel disease, rectal polyps, and diabetes mellitus. CEA is usually elevated only mildly in most of these benign conditions. Therefore, serial CEA determinations after surgical removal of colorectal cancer are still an important test for recurrent disease. Marked elevation of CEA reliably indicates recurrent or persistent cancer in most cases but does not provide anatomic localization of the tumor. Elevated CEA levels in a patient with colon cancer indicate a search for a site of recurrent disease. A serum CEA concentration greater than 9 ng. per ml. combined with a positive result of a liver imaging test predicts metastases with 98% accuracy. Conversely, a normal CEA value combined with a negative hepatic imaging test result is about 95% accurate in excluding hepatic metastases from colorectal cancer. An elevated serum alkaline phosphatase concentration is reported to have a 75% sensitivity in the detection of hepatic metastases, and the combination of alkaline phosphatase and CEA has a sensitivity of nearly 90%, with a false-positive rate of about 10%. Other blood tests that can reflect the presence of hepatic metastases include determination of levels of gamma-glutamyl transpeptidase, 5'-nucleotidase, alanine transaminase, and lactate dehydrogenase. The lactate dehydrogenase study is reported to have the highest sensitivity (85%) of all standard liver function tests but also the highest false-positive rate (about 50%). The latter observation illustrates the difficulty in using routine liver function tests alone in surveillance for recurrent disease. A prospective study by the National Institutes of Health concluded that laboratory tests alone were inadequate for detecting liver metastases. The consensus group recommended a single liver imaging study plus selected blood tests as a screening protocol for liver metastases for colorectal cancer. The imaging studies most commonly used for this purpose are ultrasonography, CT, and MRI. Each imaging test has its advantages and disadvantages. Ultrasonography is relatively inexpensive but operator dependent. It is highly accurate in the characterization of certain liver masses such as cysts and permits easily guided liver biopsies. The CT scan is generally considered the most favored test because of its availability and overall accuracy in the detection of liver metastases. The sensitivity of CT varies widely according to the technique, the experience of the radiologist, and the generation of equipment used. The two most common techniques involving CT are bolusdynamic CT and CT portography. In one study of bolusdynamic CT, liver metastases were found in 93% (26 of 28) of patients with metastatic disease and 92% (86 of 93) of tumor nodules were detected. CT portography uses knowledge on the differences in blood supply between normal liver parenchyma and liver metastases. Whereas liver parenchyma normally receives 75% of its blood supply from the portal inflow, liver metastases derive nearly all their vascular inflow from branches of the hepatic artery. The same is true of most primary tumors of the liver. The procedure is done in two phases—a conventional arteriogram and then a CT scan performed during the portal venous phase of a superior mesenteric arterial or splenic arterial injection. Therefore, lesions show up as dark spaces. CT portography is by far the most sensitive available imaging test but also one of the least specific and most expensive. The sensitivity in detecting lesions as small as 5 mm. in diameter is about 97%. However, many of the lesions need correlation with ultrasonography, MRI, or open surgical techniques. The technique also requires a large volume of injected contrast material and therefore considerable periprocedural patient hydration. A distinct alternative for potentially resectable lesions is to bypass CT portography and go directly to laparoscopic exploration with ultrasonography before a traditional incision. The latter approach is less expensive and possibly more efficient. A variety of techniques are being developed that use MRI. T1-weighted images are used primarily for determination of anatomy. T2-weighted sequences remain the most useful, plus there are some developments that show great promise for detection of pathologic processes. Gadolinium-enhanced techniques are probably useful for both detection and characterization of liver lesions. Fast spin-echo and gradient refocusing are reducing scan times and expense. MR cholangiography and various arterial and portal flow techniques are also showing great promise. In contrast, nuclear scans have lost most of their practicality because of improvement in the sensitivity and accuracy of other tests. Various antibody tests to CEA and other tumor markers have developed but not achieved great reliability. The latter test may have limited usefulness for the patient with a markedly elevated serum CEA value but no metastasis detectable by other modalities. Treatment Resection, if possible, is the treatment of choice for metastatic colorectal cancer to the liver. Liver lesions detected on careful follow-up will often be resectable, and several studies have documented the unfavorable prognosis of untreated hepatic metastases from colorectal cancer. Without treatment, 60% to 70% of patients die within 1 year and close to 100% have died within 3 years. Although earlier reports suggested that patients with untreated solitary liver metastases did not live much longer than those with multiple tumors, some critical analyses have demonstrated small but significant differences in survival among patients based on the number of metastases. No doubt the natural history of patients with hepatic metastases is variable. However, rarely does an individual with unresected disease live more than 3 years with the diagnosis of metastatic liver cancer. Resection of a solitary metastatic lesion from a colorectal primary tumor can have as much as a 40% 5-year survival rate. When a synchronous hepatic metastasis is found during operation with a primary tumor in colorectal malignancy, the hepatic lesion may be removed simultaneously or at a second procedure. The decision is based on the adequacy of colon preparation, magnitude of the principal procedure, anticipated extent of hepatic resection, general status of the patient, and experience of the surgeon. Because no randomized prospective studies have been performed, it has not been proved whether the prolongation of survival after resection of hepatic metastases is a function of the resection or the natural biology of the tumor. With the improved safety of hepatic resection, the indications for hepatic resection of metastases have expanded. Examples include resection of large symptomatic lesions in the presence of extrahepatic disease and resection of residual hepatic disease in combination with aggressive chemotherapy. In general, contraindications to major hepatic resection for metastatic disease include total hepatic involvement, advanced cirrhosis, jaundice (except from extrinsic hepatic ductal obstruction), vena cava or main portal vein invasion, and extrahepatic tumor involvement. Transplantation of the liver for metastatic disease has generally been unsuccessful except for select tumors such as carcinoid. A review of 345 patients who underwent surgery for hepatic metastases revealed that 70% had primary colorectal lesions. Wilms' tumor, melanoma, and leiomyosarcoma represented another 10%, and a variety of other tumors made up the remaining 20%. The cumulative 5year survival rate after hepatic resection of colorectal metastases was 22%. Patients with tumors more than 5 cm. in diameter had a poorer outcome. Survival did not correlate with the time interval between resection of the primary tumor and resection of the metastatic tumor, nor with the extent of liver resection. No difference in survival was found between patients with synchronous and those with metachronous lesions or between patients with solitary lesions and patients with multiple lesions in the same lobe. In addition, the status of colonic lymph node involvement in the resected primary tumor had no effect on survival. Data are beginning to indicate that a number of factors are predictors of survival after resection of colorectal hepatic metastases. These include size, number of metastases, and presence of residual local disease. Logic, but little data, suggests that the colonic lymph node status should be another predictor. Interestingly, several studies suggest that females have a better survival after hepatic resection of colorectal hepatic metastases. Other Treatment Modalities. A variety of other treatment modalities have been advocated for treatment of metastatic colorectal cancer to the liver. The chemotherapeutic agent 5- fluorouracil has about a 20% reported response rate when given systemically for treatment of tumors of the gastrointestinal tract. However, the response appears to be even less in the presence of hepatic metastases. Leucovorin or levamisole appears to add to this rate. A hepatic artery infusion pump (Infusaid) is used in several centers. Failure to accrue enough patients for large studies of the pump in the mid 1980s led to general pessimism about the use of the pump for treatment of colorectal metastases. Seven randomized trials now have demonstrated higher partial and complete response rates of intrahepatic versus systemic infusion in the treatment of hepatic metastases from colorectal carcinoma. The toxicity of direct hepatic arterial therapy is surprisingly low. A rare and irreversible problem is severe biliary sclerosis, which is related to biliary ischemia. Cryoablation, hyperthermia, and various chemoembolic hepatic artery occlusion techniques continue to be investigated in several centers. Chemoembolization has had its best success with metastatic leiomyosarcoma. Radiation therapy has had no real success in the treatment of hepatic metastases. Resection of Noncolorectal Metastases. Neuroendocrine malignancies, including metastatic carcinoid, represent the second most common indication for resection of secondary liver tumors. About 80% of patients who undergo resection with metastatic neuroendocrine tumors are symptomatic. Ninety percent of such patients with carcinoids have an endocrine syndrome. The primary indication for resection in most cases is palliation. The chance of cure with resection is small. Reduction of tumor volume by resection or cryoablation results predictably in elimination or amelioration of symptoms, often for years. There has usually already been a failure of medical management of the syndrome, such as after octreotide (Sandostatin) therapy or chemotherapy. Hepatic metastases from primary malignancies other than colorectal and neuroendocrine (including lung, breast, stomach, pancreas, melanoma, and the rest of the gastrointestinal tract) connote dismal outcomes. Solitary metastases from these lesions are rare, probably related to the natural biology of these tumors. However, data on aggressive, combined medical/surgical treatment are sparse. Rare favorable results with liver resection have also been reported with Wilms' tumor, renal cell carcinoma, gallbladder cancer, bile duct cancer, and even adrenal cancer. Long-term survival has not been reported after resection of melanoma nor pancreatic cancer. The authors have treated 20 patients with residual liver deposits of breast cancer after bone marrow transplantation chemotherapy, and more than half the patients have survived well over a year, suggesting a benefit. BENIGN HEPATIC NEOPLASMS Benign tumors of the liver are relatively common, occurring in about 1% of autopsies. CT and MRI are considerably more sensitive than autopsy and find some kind of liver lesion in about 5% of patients. Benign tumors may be classified into true neoplasms, hamartomas, and pseudotumors. The nonbiliary neoplasms of the liver can also be classified as hepatocellular, vascular, and other nonvascular lesions. This section discusses the most common benign lesions with their characteristic features. Adenomas and Focal Nodular Hyperplasia Liver cell adenoma and focal nodular hyperplasia are frequently difficult to differentiate, although each has its own distinct pathologic and clinical features. The striking similarities are that both occur primarily in young women and are associated with the use of oral contraceptives and that pathologically both are composed of hepatocytes. Hepatic adenomas are usually solitary and may vary in size up to 38 cm. in diameter. Occasionally they may be multiple and cluster within families. They are prone to hemorrhage and necrosis, and tumor rupture or dramatic bleeding occurs in approximately one third of patients. Malignant change is possible. The remaining patients present because of pain or a palpable mass. The patient with an unresected adenoma who discontinues using oral contraceptives and becomes pregnant is at considerable risk for tumor rupture and hemorrhage. Microscopically, the adenomas are closely approximated cords of hepatocytes that have vacuolated sinusoidal borders . Centers of the adenomas may undergo degenerative changes. Adenomas have an abundant blood supply; the benign tumor appears separate from adjacent normal hepatic tissue. In contrast, focal nodular hyperplasia does not produce symptoms; and hemorrhage, rupture, or other problems, such as malignant change, are exceedingly rare. Histologically, this lesion has a central stellate scar and no true encapsulation, and the cells, which are slightly different in color, usually blend with the normal hepatic parenchyma. The ultrastructure of the hepatocytes in nodular hyperplasia is similar to that of normal hepatocytes. The blood supply to areas of focal nodular hyperplasia is quite different from that to hepatic adenomas, with most of the supply arising centrally rather than peripherally. Nodular Regenerative Hyperplasia Nodular regenerative hyperplasia is, by definition, a noncirrhotic diffuse hepatocellular process characterized by multiple nodules in intervening areas of hepatic atrophy. It is similar to focal nodular hyperplasia in that both are not true neoplasms. Nodular regenerative hyperplasia is more frequently confused with cirrhosis. Both are frequently associated with portal hypertension, but the former is distinguished by the absence of severe fibrosis. Although this condition is generally considered rare, because of the understandable confusion between this entity and cirrhosis the incidence is reported quite variably. Cavernous Hemangiomas Cavernous hemangiomas occur in all age groups, often grow during pregnancy, and are usually successfully diagnosed by ultrasonography, CT, MRI, radionuclide scan, or arteriography . Approximately 2% of livers at autopsy contain cavernous hemangiomas, making this the most common liver tumor encountered coincidentally at laparotomy. Most hemangiomas are small and do not cause symptoms. However, they may be large and when associated with diffuse hemangiomatosis can nearly replace the liver. Spontaneous rupture is unusual but can be dramatic. Rare complications include congestive heart failure due to arteriovenous shunts and consumptive coagulopathy. Biopsy of a cavernous hemangioma may lead to severe, uncontrollable hemorrhage. Indications for resection are usually determined by the presence of symptoms, the danger of rupture, and the amount of liver tissue involved. Symptoms usually indicate enlargement and are associated with increased incidence of rupture. The occupation of the patient and size of the lesion influence the decision to resect. For example, a large lesion in a professional football player should be removed. On the other hand, most hemangiomas do not have to be treated. Other Benign Solid Tumors Other benign solid tumors that may appear in the liver include lipomas, fibromas, leiomyomas, myxomas, teratomas, carcinoid tumor, and mesenchymal hamartomas. Carcinoid is an exceedingly rare primary liver tumor and is associated with the carcinoid syndrome. Mesenchymal hamartomas are rare but important to recognize because they grow to an extremely large size in an infant or young child and require surgical resection. Biliary cystadenomas and bile duct adenomas are exceedingly rare and may cause pain or extrahepatic biliary obstruction. Other even rarer benign biliary tumors include meningioma, fibroma, granular cell myoblastoma, and carcinoid. Tiny biliary hamartomas, or tufts of biliary hyperplasia, are extremely common. Another lesion usually of little pathologic significance but relatively common is focal fatty change. Some benign conditions that can be confused with hepatic neoplasms include hereditary hemorrhagic telangiectasia, peliosis hepatis, and hepatic pseudotumor. Hereditary hemorrhagic telangiectasia is a diffuse telangiectatic process in the liver with numerous arteriovenous fistulas; it is rare, associated with fibrosis, and considered by some authorities to be a form of cirrhosis. Peliosis hepatis is also a rare lesion characterized by variably sized blood lakes and the most common association is with anabolic steroid therapy. Rarely is the condition clinically important. Over 50 cases of inflammatory pseudotumor have been reported, most probably resulting from healed abscesses. NONPARASITIC CYSTS Cysts of the liver are generally benign. They may be solitary or multiple and may or may not communicate with the hepatic ductal system. Large solitary parenchymal cysts appear to be very rare; only 48 have been observed by investigators at a major medical center in the past 30 years. Most cysts are lined by biliary epithelium but may also have a mesothelial cell lining or rarely other types of lining. They are more common in the right lobe of the liver. It has been presumed that most of these cysts are congenital. They occur four times as frequently in females as in males. Although most cysts are small and asymptomatic, they may be quite large; in fact, one reported cyst contained 17,000 ml. of fluid. The most common presenting symptoms of large cysts are increased abdominal girth, vague pain, occasional bleeding or infection, and rarely evidence of significant hepatocyte compromise such as obstructive jaundice. Small cysts require no treatment, although if they are discovered incidentally at the time of operation they may simply be aspirated. For large cysts from which clear fluid is aspirated, the preferred treatment is excision. However, major vascular and ductal structures may be proximal to the wall, in which case unroofing and external drainage is the treatment of choice. Many of these excisions have been performed laparoscopically at several centers. An infected cyst should be treated like an abscess, that is, with open drainage. If the cyst contains biliary contents, a communication to the bile duct system should be presumed and excision or Roux-en-Y cystojejunostomy is the treatment of choice. Polycystic liver disease often accompanies polycystic kidneys. The number of cysts varies, but most do not cause liver function compromise; and rupture, hemorrhage, and infection are rare. When indicated, they may be treated in a manner similar to solitary cysts. Injection of sclerosing solutions such as tetracycline and formalin has been performed with only limited success. Simple aspiration is a temporizing maneuver. The surgical procedure of choice for a symptomatic dominant cyst in polycystic disease is the fenestration operation, in which the symptomatic cyst is made to communicate with the peritoneal cavity. Cysts may also form as a consequence of trauma or inflammation; however, these are not true cysts, because they have a fibrous rather than an epithelial lining. There are no special aspects of treatment of these cysts. Cystadenomas, cystadenocarcinomas, and other neoplasms with necrotic centers obviously may present as cysts and should be treated by excision. Cystadenoma is thought to be a precursor of cystadenocarcinoma. Despite the rarity of cystadenoma, there have been several large series of resection of cystadenomas. This disparity suggests there may be some confusion in the diagnosis of this lesion. Other unusual cysts include teratomas, necrotic cysts secondary to infarction, intrahepatic duodenal duplications, and cysts associated with congenital hepatic fibrosis. Peliosis hepatis is a dilation of the hepatic sinusoids often associated with steroids, chemotherapy, and tuberculosis. Choledochal or other solitary ductal cysts are more common in Asia than in the United States. They occur more commonly in females than in males, but the pathogenesis is unknown. Sixty percent are diagnosed before the age of 10. Associated intrahepatic ductal dilation is frequent. The most common type of choledochal cyst is one that involves the common bile duct and cystic duct but does not involve the junction of the common hepatic duct. A number of other types may occur, including a diverticulum, diffuse biliary ductal involvement, and segmental dilation. Treatment of choledochal or hepatic ductal cysts or dilation is surgical and is supplemented by antibiotics to control infection. Because of the significant likelihood of biliary obstruction, cholangitis, calculi, and carcinoma, the procedure of choice is excision whenever possible. However, this may not be practical anatomically. In addition, the drainage procedures such as hepaticoenterostomy or cystoenterostomy are preferred. The incidence of carcinoma in congenital biliary duct cysts appears to be 5% to 8%. Occasionally this occurs after cyst excision. The incidence of stricture after cystoenterostomy has been reported to be as high as 40% to 50%. Multiple cystic dilations of the intrahepatic ducts (Caroli's disease) are a congenital malformation often associated with congenital hepatic fibrosis. It may be confined to one segment or lobe but is usually diffuse. This problem also appears to be more common in Asia. Symptoms of biliary tract disease, such as colic or cholangitis, may be associated with Caroli's disease. Treatment depends on the location and extent of the intrahepatic ductal dilation. Both drainage and resection in selected patients have been reported to provide good results. ECHINOCOCCAL CYSTS Echinococcosis, or hydatidosis, is the most frequent cause of liver cysts in the world. The problem is endemic in Greece, other parts of Eastern Europe, South America, Australia, and South Africa. Echinococcosis is rare in the United States, although it is prevalent enough to be seen by most general surgeons during their careers. The most common form is due to Echinococcus granulosus, although occasionally E. multilocularis is the infective agent. The adult E. granulosus is a tapeworm that resides in the jejunum of dogs. Eggs are passed in the stool and ingested by cows, sheep, moose, caribou, or humans. Embryos pass through the intestinal mucosa into the portal circulation and are filtered by the liver and occasionally by the lungs. They then develop into cysts that have two layers, an outer fibrous layer and an inner parasite-derived layer. The inner layer is the germinal membrane that contains the scolices and daughter cysts and may float freely in the clear cyst fluid. Approximately 80% of hydatid cysts are initially single and in the right lobe. The most common presenting symptoms or signs are abdominal pain and palpation of a mass in the right upper quadrant. The cysts are usually greater than 5 cm. in diameter when they cause symptoms. The complications of echinococcal cysts include infection, rupture, anaphylaxis, biliary obstruction, and liver replacement. The patient may have eosinophilia and mildly elevated results of liver function tests. Of the serologic tests, the indirect hemagglutination test and the Casoni skin test have approximately an 85% sensitivity. Problems with the Casoni skin test are that the test itself may sensitize the host, which may cause false-positive serologic tests or even anaphylaxis, and there is a high frequency of false-positive tests due to poor standardization of the nitrogen content in the antigen. The complement fixation test has approximately a 70% sensitivity. Calcification of the cystic wall is present in over half the patients. Liver scan, ultrasonography, CT, and arteriography all can have nearly 100% sensitivity. Endoscopic retrograde cholangiopancreatography and cholangiography have been reported to be helpful occasionally. The finding of daughter cysts or hydatid sand on ultrasonography and CT helps differentiate this cyst from pyogenic or amebic liver abscess. This entity must be suspected to avoid percutaneous needle aspiration, which may cause spillage and spread of the cysts. Communication of the cyst with the biliary tract may be seen on the preoperative cholangiogram or intraoperatively in approximately one fourth of patients. Treatment is primarily surgical. At exploration, the abdomen is carefully packed with pads around the cysts to reduce the risk of peritoneal soilage. The cyst may then be aspirated as completely as possible with a closed system. If the fluid color suggests biliary communication, a sclerosing solution should not be used. Hypertonic saline, chlorhexidine, 80% alcohol, and 0.5% cetrimide are all useful as scolecidal agents and may be instilled into the cyst cavity. Formalin is no longer used because of the risk of systemic toxicity. After 5 minutes, the procedure is repeated and then the cyst cavity is unroofed. The fluid in the echinococcal cyst cavity is highly antigenic and therefore anaphylaxis as well as spread is a risk of cyst rupture. Instillation of scolecidal agents is effective in destroying 80% to 90% of scoleces. There are two alternatives in the management of the cyst cavity: drainage and obliteration without drainage. Except in cases with biliary communication, drainage is accompanied by greater postoperative morbidity and prolonged hospitalization. Therefore, most surgeons prefer not to drain the cyst cavities. Small intrahepatic or extrahepatic cysts may be excised. Recently, mebendazole has been found to be an effective agent in some cases of echinococcal cysts, and this drug may be used for cases not amenable to surgical therapy. The treatment of the more aggressive E. multilocularis, or alveolar hydatid disease, is excision whenever possible. However, this is rarely possible. This disease is limited geographically to the Northern Hemisphere, being endemic in Alaska and Canada. Natural hosts include the fox, coyote, and small rodents. MAJOR HEPATIC RESECTION With reduction of elective operative mortality to less than 1%, major hepatic resection has gained wide acceptance as the primary therapy for many primary and metastatic tumors to the liver and other selected benign conditions. Those conditions include segmental Caroli's disease or hepatolithiasis, echinococcosis, or solitary hepatic abscesses in chronic granulomatous disease of childhood. Formerly, only four types of major hepatic resection were employed, based on the lobar (American) system of anatomy: (A) right hepatic lobectomy, (B) left hepatic lobectomy, (C) right trisegmentectomy, and (D) left lateral segmentectomy . Now, a number of new resections have become commonplace in major centers. Most are based on the French segmental system. Basically, there are three types of hepatic resection: (1) anatomic resection, (2) enucleation operation, and (3) nonanatomic resection. The primary concept behind anatomic resection according to the segmental anatomy is that malignant cells distribute along the portal venous segmental supply. Enucleation operations are used for specific benign lesions with limited chance of local invasion (e.g., hemangioma, developmental cysts, focal nodular hyperplasia, or even hepatic adenoma). Nonanatomic resections are appropriate for pathologic processes in which a limited margin is appropriate (e.g., echinococcal cysts and abscess excision in chronic granulomatous disease). The techniques of resection are distinguished by the presence or absence of partial or complete vascular occlusion. Vascular occlusion has the advantage of reduction of blood loss and the disadvantage of ischemic injury relating to the duration of occlusion. Most experienced surgeons use vascular occlusion selectively, depending on the individual cases. At Duke University Medical Center, a selective approach with respect to occlusion is used and about half the patients require blood transfusion. The techniques of parenchymal dissection have been greatly facilitated by the development of various instruments, such as the argon beam coagulator and the Cavitron ultrasonic aspirator. The latter instrument has a metal tip that vibrates at an ultrasonic frequency that fragments and detaches parenchymal cells. An irrigation port lifts the cells so that a suction port can remove them. Major biliary or vascular structures remain intact, permitting their isolation and ligation, even without hilar dissection. Through the use of these and other techniques, many more lesions can be safely resected than was formerly believed and partial hepatic transplantation has become possible. A variety of incisions may be used for hepatic resection. Most surgeons stay below the diaphragm with a right subcostal incision and a left subcostal extension if necessary. The incision can be extended as a median sternotomy or right thoracotomy. A paramedian incision is preferred by many surgeons in Asia. Lobectomy and trisegmentectomy usually require hilar dissection, whereas segmental resections generally do not. Branches of the portal vein, hepatic artery, and bile duct of the lobe to be removed are ligated and divided. The hepatic veins may be ligated extrahepatically or during the parenchymal dissection. Parenchymal dissection is performed with a small clamp, handle of the knife, or finger fracture. A variety of noncrushing liver clamps may be applied to control parenchymal bleeding, and surgical staplers are useful during the latter stages of the parenchymal dissection. Compulsive preoperative hydration is key to a benign postoperative course. Dehydration leads to rapid intraoperative volume resuscitation, which leads to hepatic venous congestion, the chief cause of morbidity and mortality. Hypoglycemia and hypoproteinemia frequently occur in the postoperative period after major hepatic resection. These should be treated supportively and usually resolve within 1 week after the operation. Alkaline phosphatase and transaminase elevations frequently occur, and transient hyperbilirubinemia is not uncommon. Up to 80% or 90% of the liver may be removed without serious consequence if the remaining liver is normal. Patients with pre-existent diffuse liver disease are at greatly increased risk for liver failure after resection. Regeneration is nearly complete by 3 weeks postoperatively, except in elderly patients, whose livers grow back more slowly. IV. HEMOBILIA HEMOBILIA Hemobilia, a term introduced by Sandblom in 1948, refers to a relatively common and occasionally severe clinical problem. A severe case should engender in the clinician certain reflex responses with respect to diagnosis and treatment. Glisson provided the first modern description of bleeding within the biliary tract in 1654, recognizing that it was probably a relatively common consequence of trauma. Rupture of a hepatic artery aneurysm into the biliary tract was first reported by Jackson in 1921. In 1948, Sandblom recognized the classic triad of pain, jaundice, and hematemesis. With severe bleeding, blood usually moves in both directions in the gastrointestinal tract, causing both hematemesis and melena. Recently, the more common causes of identifiable hemobilia reflect advances in hepatobiliary diagnostic and therapeutic techniques. These include percutaneous liver biopsy, transhepatic or endoscopic cholangiography, extracorporeal lithotripsy, transjugular intrahepatic portosystemic shunt, and laparoscopic cholecystectomy. ETIOLOGY Bleeding may arise anywhere within the biliary system, that is, from liver parenchyma, intrahepatic or extrahepatic bile ducts, gallbladder, pancreas, or the ampullary region. The communication between the vascular and biliary systems may be caused by laceration, pressure necrosis, tumor, or infection. In addition, thrombolysis in bile may contribute to continued bleeding. Because of its higher pressure, the arterial system is more often involved than the venous system. In earlier reviews, operative trauma represented approximately 15% of cases of hemobilia. Other causes include major procedures on or near the liver that may produce minor or major bleeding, such as liver, stomach, or colon resections; cholecystectomy; or extraction of intrahepatic calculi . The procedure most likely to cause bleeding within the biliary system is common bile duct exploration. The peribiliary arterial plexus is often involved in the bleeding. Hepatic artery false aneurysms may follow unsuspected injury and later erode into the bile duct. Postoperative dislodgment of a T-tube may also produce the problem. The diagnosis of thrombus within the biliary tree is easily made by cholangiography if a catheter such as a T-tube is within the common bile duct. Thus, thrombus may occasionally be confused with a retained stone. Recently, percutaneous cholangiography has become an important cause of bleeding into the biliary system. Hemobilia occurs after 4% of percutaneous transhepatic cholangiograms and 9% of percutaneously placed biliary drainage catheters. Some degree of hemobilia occurs in 5% to 10% of therapeutic endoscopic procedures. In these patients, thrombus in the biliary tree usually evolves from the sphincterotomy site. Some degree of hemobilia occurs in 8% to 14% of extracorporeal hepatic biliary lithotripsy and 4% to 8% of extracorporeal gallstone lithotripsy. In nearly all of the latter cases the reported bleeding has been minor and inconsequential. In a review of 545 patients in 1972, trauma was the most important causative factor in 48% of cases, followed by infection in 28%, gallstones in 10%, aneurysms in 7%, and tumor in 5%. Trauma included both blunt and penetrating injuries, with that resulting from vehicular accidents leading the list. Blunt trauma may cause deep liver injury with or without disruption of Glisson's capsule. Probably the most frequent reason for hemobilia occurring after liver injury is bleeding deep within the substance of the liver when the capsule remains intact, is sutured closed, or heals superficially. If the resultant hematoma expands, it may rupture into the biliary system; or a false aneurysm may occur and lead to the same problem in a delayed manner. These are the principal reasons that suture closure of a liver laceration is generally not recommended. Penetration may cause hemobilia by a similar mechanism or by creating a direct communication of a vascular structure with the biliary system. Infection may also be an important factor in the development of hemobilia. In the Far East, the parasites Clonorchis sinensis and Ascaris may cause cholangitis or pericholangitic abscesses, which may cause hemobilia. Less commonly, amebic abscess, tuberculosis, and Echinococcus infestation are implicated. Biliary tract infection in North America is more often caused by calculi, which may also cause hemobilia by direct trauma to the ampulla or other sites within the duodenal ductal system. Before the development of oral cholecystography, the coexistence of biliary colic with blood in the stool was considered a reliable sign of gallstones. The term hemocholecyst refers to bleeding within the gallbladder, which rarely may be the cause of biliary colic. Pyogenic liver abscess may lead to hemobilia by direct erosion into a vessel, by formation of a pseudoaneurysm, or as a complication of treatment. Hemobilia may also follow a mycotic aneurysm complicating subacute bacterial endocarditis or another disease process. Pancreatitis may cause hemobilia by erosion of a large vessel such as the splenic or gastroduodenal artery and communication with the pancreatic duct; the outcome is usually fatal. Hemobilia due to pancreatic disease is unusual except when cancer has, by erosion, entered the biliary system. Hemobilia can also occur as a manifestation of rupture of an echinococcal or amebic abscess. Various aneurysms may cause hemobilia by pressure necrosis. Arteriosclerotic, congenital, false, or mycotic aneurysms of the hepatic or gastroduodenal artery have all been reported to cause this problem. In one series, 43 of 103 ruptured hepatic artery aneurysms (42%) led to hemobilia. Hepatoma secondary to cirrhosis is the most common tumor causing hemobilia. Other tumors that have been reported to induce hemobilia include gallbladder cancer, cholangiocarcinoma, hemangioma, angiosarcoma, adenomas, cystadenomas, cystadenocarcinomas, and metastatic lesions of the liver parenchyma, gallbladder, and bile ducts. Hemobilia may also occur in association with choledochal cyst or may appear spontaneously in association with vasculitis, heterotopic gastric mucosa, or hemolytic disease such as sickle cell anemia. In the authors' unpublished review of hemobilia during the past 3 years, 24 of 43 cases were the direct result of iatrogenic trauma, 12 were postsurgical, 6 followed laparoscopic cholecystectomy, and 2 were complications of cryoablation of liver masses. Others were caused by complications of transcatheter arterial embolization, alcohol injection of liver lesions, transjugular intrahepatic portosystemic shunt, percutaneous liver biopsy, lithotripsy, endoscopic sphincterotomy, percutaneous abscess drainage, and transhepatic cholangiography. Twelve patients had hemobilia as the initial presentation of a hepatobiliary tumor and 8 of the 12 tumors were resected. SYMPTOMS AND SIGNS The classic triad of hemobilia comprises gastrointestinal bleeding, right upper quadrant pain, and jaundice. These features may also suggest other diseases, including terminal cancer. In the setting of trauma, general good health, or any type of hepatobiliary manipulation, this triad should suggest the possibility of hemobilia. All three symptoms are not necessarily present. Acute bleeding usually first causes pain, followed by hematemesis or melena. Hemobilia may occur days, weeks, or months after trauma. A palpable mass representing the liver or gallbladder may accompany these symptoms. When a right upper quadrant bruit is heard with the classic triad, a visceral artery aneurysm causing hemobilia should be considered. DIAGNOSIS Early diagnosis may be lifesaving with severe hemobilia. A pseudoaneurysm should immediately be suspected, and arteriography remains the single most accurate and helpful diagnostic test. Liver or biliary scans, ultrasonography, computed tomography, or magnetic resonance imaging may provide helpful information such as evidence of anatomic defects or abnormalities in the liver or bile ducts. Endoscopic retrograde cholangiopancreatography or percutaneous transhepatic cholangiography may be helpful, but these procedures, particularly the last one, may confuse the diagnosis because this manipulation may also create a new source of bleeding. Ultrasonography may yield an erroneous impression of normal duct size in the presence of fresh blood or clot because of its variable echogenicity. Technetium-labeled or another red blood cell scan may occasionally be an efficient way of establishing a diagnosis, particularly in stable patients, but may also delay definitive diagnosis and treatment in severely bleeding patients. An external biliary drainage catheter may demonstrate the bleeding, or injection of the catheter with contrast material may document thrombus within the biliary system. Immediate surgical therapy is rarely required to establish the diagnosis of severe hemobilia, although it may occasionally be indicated. If the operation is performed before diagnosis, a cause such as hepatic artery aneurysm, calculi, or tumor may be evident, or the biliary system may be the site of a collection of blood. MANAGEMENT The conditions associated with hemobilia can cause severe blood loss and, in some cases, hemorrhagic shock. Initial management of the patient should be general evaluation and resuscitation, with blood transfusions as needed. Careful monitoring with blood replacement is important in the care of these patients. If anticipated, coagulopathy may be prevented or managed by administration of blood components such as fresh frozen plasma or platelets. After the diagnosis of hemobilia is established, there are several options for treatment. The majority of cases of hemobilia, particularly those involving intrahepatic false aneurysms, may be effectively treated with arteriographic techniques such as embolization with clot, steel coils, or other emboli. Selection of operative versus nonoperative treatment is based on the severity of bleeding, the underlying cause, and the age and general state of the patient. If the bleeding appears to be minor and easily monitored (e.g., in a postoperative situation), it is best managed expectantly. With acute trauma and hemoperitoneum, management of the acute bleeding is a primary concern. Hepatic parenchymal bleeding may be controlled by a variety of methods, including direct ligation of the bleeding vessel and resection, packing, or ligation of the proper, common, right, or left hepatic artery. Ligation of the proper hepatic artery rarely causes any clinical problem because of abundant collateral vessels. Ligation of the common, right, or left hepatic artery more often causes enzyme abnormalities but is usually tolerated by the patient, particularly if it is a lifesaving maneuver. Cholecystectomy is curative with bleeding into the gallbladder. A false aneurysm within the parenchyma of the liver is best treated nonoperatively by embolization of the involved arterial branch or balloon tamponade with arteriographic control. Underlying processes, such as biliary calculi, infection, or tumor are usually best managed simultaneously with control of hemobilia. If a common bile duct exploration is performed, selective occlusion of the right or left hepatic artery may indicate which system is involved in the bleeding. In addition, selective occlusion of the right and left hepatic ducts may determine which duct is bleeding after extraction of multiple intrahepatic calculi. In selected cases, moderate to severe bleeding in children has been treated by vigorous supportive therapy, including blood replacement and serial angiography to monitor progressive healing. Visceral artery aneurysms may be treated operatively by ligation and usually with arterial repair. Hemobilia after percutaneous transhepatic cholangiography is usually minor and ceases spontaneously. However, it may cause several problems, including ineffective biliary drainage, difficulty in determining the diagnosis of the primary problem, or continued bleeding from the catheter into the biliary system or into the peritoneal cavity. If these problems do not resolve, operation may be indicated. All reported cases after gallstone lithotripsy have been minor; but with increased use of this technique, severe bleeding is likely to occur occasionally. The overall mortality from severe hemobilia is estimated to be between 10% and 20%, depending on the age of the patient and the underlying etiology, but mortality decreases with increased awareness of the condition and prompt, appropriate therapy. Treatment of hemobilia due to other iatrogenic causes depends on the severity of the bleeding and type of manipulation. Most bleeding of this type can be controlled with arteriography. SURGICAL COMPLICATIONS OF CIRRHOSIS AND PORTAL HYPERTENSION HISTORICAL REVIEW Cirrhosis was first described in a fourth century B.C. Hippocratic aphorism: “In cases of jaundice it is a bad sign when the liver becomes hard.” 4 Although the deleterious effect of alcohol on the liver was appreciated by Galen and his contemporaries in the second century A.D., alcoholic liver disease as an entity was first recognized by Baillie and other English writers after the “gin plague” in the eighteenth century. Shortly thereafter, Lannec introduced the term cirrhosis, which was derived from the Greek word kirrhos, meaning “orange-yellow.” Nineteenth-century European and English pathologists, including Carswell and Rokitansky, described the gross and histopathologic characteristics of the disease. Although alcoholic cirrhosis was thought to be due to toxins other than alcohol or to malnutrition during much of the twentieth century, recent investigations have established alcohol itself as a hepatotoxin. Cirrhosis is the end result of a variety of mechanisms causing hepatocellular injury, including toxins (alcohol), viruses (hepatitis B and hepatitis C), prolonged cholestasis (extrahepatic and intrahepatic), autoimmunity (lupoid hepatitis), and metabolic disorders (hemochromatosis, Wilson's disease, alpha 1-antitrypsin deficiency). Although the mechanisms are diverse, the pathologic response is uniform: hepatocellular necrosis followed by fibrosis and nodular regeneration. Each of these elements may exist alone (necrosis, uncomplicated hepatitis; fibrosis, congenital hepatic fibrosis; nodular regeneration, partial nodular transformation), but all three are required for the development of cirrhosis. Cirrhosis is always a diffuse process and may be classified either morphologically or by etiology. Alcoholic cirrhosis, which is usually micronodular, and posthepatitic cirrhosis, which is generally macronodular, are the two most common varieties in the United States. Because the pathologic response to various mechanisms of hepatocellular injury is so similar, occasionally the cause cannot be ascertained (cryptogenic cirrhosis). Cirrhosis causes two major phenomena: hepatocellular failure and portal hypertension. Even after the noxious agent is removed (e.g., abstinence from alcohol), the disease may progress. Although the mechanism is not clear, both ischemia, secondary to extensive fibrosis and intrahepatic and extrahepatic shunts, and autoimmune factors may play roles. The altered hepatic architecture and perisinusoidal fibrosis cause increased hepatic vascular resistance, resulting in portal hypertension and its associated complications of variceal hemorrhage, encephalopathy, ascites, and hypersplenism. Autopsy studies suggest an incidence of cirrhosis of between 3.5% and 5.0%. Only 15% of heavy drinkers develop alcoholic cirrhosis. However, because of the large number of alcoholics in the United States, as well as a significant percentage of patients with nonalcoholic causes of chronic liver disease, cirrhosis presently ranks as the sixth leading cause of death between the ages of 35 and 54 for both males and females. Hepatic failure and variceal hemorrhage are the first and second most common causes of death, respectively, in patients with cirrhosis. Historically the treatment of cirrhosis has been the treatment of the complications of portal hypertension. Presently, medical treatment of cirrhosis with antifibrogenesis drugs such as colchicine and penicillamine is experimental. In contrast, since 1980 the surgical management of chronic liver disease with hepatic transplantation has been highly successful with long-term survival rates generally above 70%. A major challenge to the physician or surgeon managing patients with cirrhosis is to determine when definitive treatment (transplantation) rather than palliative treatment (e.g., operations to prevent recurrent variceal hemorrhage) should be applied. ANATOMY, PHYSIOLOGY, AND PATHOPHYSIOLOGY OF PORTAL HYPERTENSION The liver is a unique organ in that it has a dual blood supply: portal venous and hepatic arterial. The portal vein is formed from the confluence of the superior mesenteric and splenic veins. The left gastric or coronary vein drains the distal esophagus and lesser curvature of the stomach, generally entering the portal vein near its origin. The splenic vein lies beneath the pancreas and is usually joined by the inferior mesenteric vein just before its confluence with the superior mesenteric vein. The hepatic artery, one of three major branches of the celiac axis, lies medial to the common bile duct and portal vein in the hepatoduodenal ligament. Common variations include origins of the right and left hepatic arteries from the superior mesenteric artery and of the left gastric artery, respectively, both of which occur in nearly 20% of the population. Hepatic blood flow averages 1500 ml. per minute, which represents approximately 25% of the cardiac output. The portal vein contributes two thirds of the total hepatic blood flow, while hepatic arterial perfusion accounts for over one half of the liver's oxygen supply. The volume of portal venous flow is indirectly regulated by vasoconstriction and vasodilation of the splanchnic arterial bed. In contrast, hepatic arterioles respond to circulating catecholamines and sympathetic nervous stimulation; thus, hepatic arterial flow is directly regulated. However, even intense vasoconstrictive influences can be overcome by a hepatic arterial autoregulatory or buffer response, which maintains total hepatic blood flow as near to normal as possible when portal perfusion is decreased in patients with shock or in those with either disease-induced or surgically created portosystemic shunts. 23 Many splanchnic hormones are important regulators of hepatic metabolism. Insulin is particularly important because it is a hepatotrophic hormone and is essential for maintenance of liver structure and function. Thus, even if the quantity of hepatic blood flow is maintained in the normal range by hepatic arterial compensation for decreased portal flow, hepatic physiology may be impaired. Because increased portal venous resistance is usually the initiator of portal hypertension, classifications of this disorder are generally based on the site of elevated resistance. However, increased portal venous inflow secondary to a hyperdynamic systemic circulation and splanchnic hyperemia is often a major contributor to the maintenance of portal hypertension. The cause of the elevated cardiac output and splanchnic hyperemia is not known, but splanchnic hormones, such as glucagon, and decreased sensitivity of the splanchnic vasculature to catecholamines probably play a role. 2 The most common cause of prehepatic portal hypertension is portal vein thrombosis, which accounts for approximately 50% of cases of portal hypertension in the pediatric age group. When the portal vein is thrombosed in the absence of liver disease, hepatopetal (to the liver) portal collateral vessels develop to restore portal perfusion. This combination is termed cavernomatous transformation of the portal vein. Isolated splenic vein thrombosis (left-sided portal hypertension) is usually secondary to pancreatic inflammation or neoplasm. The result is gastrosplenic venous hypertension, while superior mesenteric and portal venous pressures remain normal. The left gastroepiploic vein becomes a major collateral vessel, and gastric varices are generally more prominent than esophageal varices. This variant of portal hypertension is important to recognize because it is easily reversed by splenectomy alone. The site of increased resistance in intrahepatic portal hypertension may be at the presinusoidal, sinusoidal, or postsinusoidal level. Frequently, more than one level is involved. The most common cause of intrahepatic, presinusoidal hypertension is schistosomiasis. In addition, many causes of nonalcoholic cirrhosis also result in presinusoidal portal hypertension, especially early in their course. Alcoholic cirrhosis, the most common cause of portal hypertension in the United States, usually causes increased resistance to portal flow at the sinusoidal (secondary to deposition of collagen in Disse's space) and postsinusoidal levels (secondary to regenerating nodules distorting small hepatic veins). Postsinusoidal causes of portal hypertension are rare and include the BuddChiari syndrome (hepatic vein thrombosis), constrictive pericarditis, and heart failure. Rarely, increased portal venous flow alone, secondary either to massive splenomegaly (idiopathic portal hypertension) or a splanchnic arteriovenous fistula, causes portal hypertension. A portal pressure above the normal level of 5 to 10 mm. Hg stimulates portosystemic collateralization. Collateral vessels usually develop where the portal and systemic venous circulations are in close proximity. Although the collateral network through the coronary and short gastric veins to the azygos vein is the most important one clinically, because it results in formation of esophagogastric varices, other sites include a recanalized umbilical vein from the left portal vein to the epigastric venous system, retroperitoneal collateral vessels, and the hemorrhoidal venous plexus. In addition to extrahepatic collateral vessels, a significant fraction of portal venous flow passes through both anatomic and physiologic (capillarization of hepatic sinusoids) intrahepatic shunts. As hepatic portal perfusion decreases, hepatic arterial flow generally increases (buffer response). 23 EVALUATION OF THE PATIENT WITH CIRRHOSIS Key aspects of the assessment of an individual with suspected chronic liver disease or one of the complications of portal hypertension are the following: (1) diagnosis of the underlying liver disease, (2) estimation of functional hepatic reserve, (3) definition of portal venous anatomy and hepatic hemodynamic evaluation, and (4) identification of the site of upper gastrointestinal hemorrhage if present. These diagnostic categories take on varying levels of importance depending on the clinical situation. For example, estimation of functional hepatic reserve is useful in determining the risk of therapeutic intervention and whether definitive (hepatic transplantation) or palliative treatment (e.g., endoscopic sclerotherapy or shunt procedure) is indicated. Knowledge of portal anatomy and physiology guides the surgeon in selecting an appropriate operation for control of variceal bleeding. Precise identification of the site of bleeding is essential because hemorrhage secondary to portal hypertension may be from esophageal varices, gastric varices, or portal hypertensive gastropathy and because a significant fraction of patients with portal hypertension bleed from other lesions. History and Physical Examination. In a patient with nonspecific constitutional complaints such as weight loss, malaise, and weakness, a past history of chronic alcoholism, hepatitis, complicated biliary disease, or exposure to hepatotoxins should lead one to include cirrhosis in the differential diagnosis. Subtle clues to the presence of underlying chronic liver disease on physical examination are spider angiomas, palmar erythema, testicular atrophy, and gynecomastia. A palpable spleen in association with these signs suggests portal hypertension. Confirmatory evidence of cirrhosis is provided by signs of hepatic functional decompensation or advanced portal hypertension such as jaundice, ascites, palpation of a firm irregular liver edge, dilated abdominal wall veins, and impairment of mental status or the presence of asterixis (liver flap). Laboratory Tests. Cirrhosis is often accompanied by anemia, leukopenia, and thrombocytopenia. Anemia may be secondary to bleeding, nutritional deficiencies, hemolysis, or bone marrow depression secondary to alcoholism. Although many patients with portal hypertension have some degree of hypersplenism, it is unusual to find a platelet count of less than 50,000 per cu. mm. or a white blood cell count less than 2000 per cu. mm. In addition to thrombocytopenia, coagulation may be impaired by a prolonged prothrombin time because many of the coagulation factors are synthesized by the liver and by primary fibrinolysis, which is present in many patients with chronic liver disease. A chemistry profile is helpful in both the diagnosis and assessment of severity of cirrhosis. Hypoalbuminemia is usually a reliable index of chronic rather than acute liver disease. Elevation of the hepatocellular enzymes, aspartate aminotransferase and alanine aminotransferase, to more than three times their normal level is indicative of significant, ongoing hepatocellular necrosis, which is often present in patients with alcoholic hepatitis and chronic active hepatitis due to a variety of causes. Increased disease activity may be an important risk factor in patients who undergo surgery. A ratio of alanine aminotransferase to aspartate aminotransferase of greater than 2 is highly suggestive of alcohol as the cause of liver disease. Although mild elevations of the enzymes alkaline phosphatase and gamma glutamyl transpeptidase are nonspecific, marked increases in these enzymes are indicative of either intrahepatic or extrahepatic cholestasis (primary or secondary biliary cirrhosis). In the absence of prior blood transfusions, a total bilirubin level of greater than 3 mg. per 100 ml. is indicative of severe hepatic decompensation and a high operative risk status. Hepatitic serology should be obtained in most patients with cirrhosis. A significant fraction of patients with hepatitis B and hepatitis C develop cirrhosis, whereas hepatitis A generally causes only acute liver disease. The most common internal malignancy worldwide is hepatocellular carcinoma, which is frequently secondary to hepatitis B infestation. However, this malignancy frequently develops in patients with other causes of cirrhosis and occasionally in patients without chronic liver disease. Unexpected hepatic functional deterioration is sometimes due to the development of hepatocellular carcinoma, which can be diagnosed in approximately 60% of patients by an elevated alpha-fetoprotein level. Common serum electrolyte abnormalities in cirrhosis are hyponatremia, hypokalemia, and metabolic alkalosis. These metabolic disorders are secondary to hyperaldosteronism, diarrhea, and recurrent emesis, which frequently accompany cirrhosis. Deleterious consequences of metabolic alkalosis are shift of the oxyhemoglobin dissociation curve to the left, which impairs tissue oxygen delivery, and conversion of ammonium chloride to ammonia, which facilitates transport of this purported cerebral toxin across the blood-brain barrier. Liver Biopsy. Percutaneous liver biopsy is a useful technique for establishing the cause of cirrhosis and for assessing activity of the liver disease. When the diagnosis is known and the chemistry profile suggests quiescent disease, liver biopsy is probably not necessary before surgical intervention for variceal hemorrhage. Percutaneous liver biopsy should not be done when either coagulopathy or moderate ascites is present. Measurement of Hepatic Functional Reserve. The time-honored method of assessing hepatic functional reserve is Child's classification . Although this classification scheme includes three clinical variables in addition to two biochemical indices and, therefore, is not a direct measure of hepatic functional reserve, no other test has surpassed it with respect to predicting operative outcome or assessing long-term prognosis in the unoperated patient. In most clinical series, operative mortality rates for Child's Class A, B, and C patients are in the range of 0% to 5%, 10% to 15%, and greater than 25%, respectively. Because many patients with acute variceal hemorrhage present with decompensated hepatic function as reflected by their Child's class, an interval of medical management to improve the patient from Child's Class C to Class A or B is worthwhile before surgical intervention. True quantitative measures of hepatocellular function, such as galactose elimination capacity, aminopyrine breath test, and hepatic clearance of amino acids, are not available in most institutions. However, these tests may be valuable indicators of limited hepatic reserve in some patients with nearly normal conventional measures of liver function. Now that hepatic transplantation has become a realistic option for many patients with cirrhosis, accurate quantitation of hepatocellular function to determine which patients are transplant candidates has become even more important. Hepatic Hemodynamic Assessment. In patients with alcoholic cirrhosis and many varieties of nonalcoholic cirrhosis, portal pressure can be indirectly estimated by measurement of hepatic venous wedge pressure. Because hepatic venous wedge pressure is normal in patients with presinusoidal portal hypertension, portal pressure in these individuals can be measured only directly by transhepatic or umbilical venous cannulation of the portal venous system or by percutaneous puncture of the spleen. However, because magnitude of portal venous pressure predicts neither likelihood of bleeding nor ultimate prognosis, the only useful application of these techniques is in differentiating between presinusoidal and sinusoidal/postsinusoidal causes of portal hypertension. Because splanchnic venous thrombosis may be the cause of portal hypertension or develop secondary to cirrhosis, portal venous anatomy should be defined before performing a portosystemic shunt operation. Selective visceral angiography has been the most frequently used method for visualization of the portal venous system and for qualitative estimation of hepatic portal perfusion. A complete angiographic study generally consists of selective injections of radiographic contrast medium into the superior mesenteric and splenic arteries followed by late venous phase films to define splenic, superior mesenteric, and portal veins. If the renal vein is to be used in shunt construction, this vessel should also be cannulated and opacified. Hepatic portal perfusion can be estimated from the venous phase of the superior mesenteric angiogram and graded as follows: Grade 1, normal perfusion; Grade 2, visualization of intrahepatic portal venous radicles; Grade 3, opacification of portal vein only; Grade 4, nonvisualization of portal vein . This grading system is particularly valuable for assessment of portal blood flow before and after a selective shunt, because one of the objectives of such a procedure is preservation of hepatic portal perfusion. Postoperative venography, either indirectly after arterial injection or directly by means of venous shunt cannulation, is the most accurate method of determining shunt patency. Duplex ultrasonography is a noninvasive alternative to angiography for assessment of portal venous patency, direction of portal flow, and shunt patency status. This technique is less accurate in assessing superior mesenteric and splenic vein anatomy and flow characteristics. Likewise, duplex ultrasonography usually accurately assesses patency status of central shunts (e.g., portacaval) but is of less value for evaluating more peripheral shunts (e.g., distal splenorenal). Diagnosis of Bleeding. In the absence of hematemesis, a nasogastric tube should be inserted to determine whether bleeding is from the upper gastrointestinal tract. The key procedure for diagnosing the site of upper gastrointestinal hemorrhage in a patient with portal hypertension is endoscopy. Before endoscopy the patient should be hemodynamically stabilized and the stomach evacuated of blood clots with a large-bore lavage tube. Upper gastrointestinal tract bleeding in patients with portal hypertension is secondary to portal hypertension in approximately 90% of instances. The remaining 10% of patients bleed from Mallory-Weiss tears, gastric ulcers, and duodenal ulcers, all of which are more common in patients with alcoholic cirrhosis than in the general population. Portal hypertensive bleeding is most commonly from esophagogastric varices (esophageal varices = 90%; gastric varices = 10%). The endoscopic diagnosis of variceal hemorrhage can be established by either observing a bleeding varix (approximately 25% of patients) or by observation of moderate- to large-sized varices and no other lesions in a patient who has recently experienced a major upper gastrointestinal tract hemorrhage (>2 units of blood). The only nonvariceal cause of portal hypertensive bleeding is portal hypertensive gastropathy. 27 The frequency of this lesion is unknown, but it is probably more common after eradication of varices by endoscopic sclerotherapy. Portal hypertensive gastropathy mainly involves the fundus and body of the stomach and has an endoscopic appearance of a white reticular network with enclosed erythematous areas. Because varices and portal hypertensive gastropathy often coexist, it may be difficult to determine which lesion is responsible for any given episode of bleeding. Occasionally, massive bleeding in a patient with cirrhosis makes an endoscopic diagnosis initially impossible, in which case endoscopy should be repeated after bleeding is controlled. VARICEAL HEMORRHAGE Bleeding from esophagogastric varices is the single most life-threatening complication of portal hypertension, responsible for approximately one third of all deaths in patients with cirrhosis. The risk of death from bleeding in any individual patient is mainly related to the underlying hepatic functional reserve. Patients with extrahepatic portal venous obstruction and normal hepatic function rarely die of bleeding varices, whereas individuals with decompensated cirrhosis (Child's Class C) face a mortality rate in excess of 25%. The greatest risk of death from variceal bleeding is within the first few days after the onset of hemorrhage and declines rapidly between then and 6 weeks, when it returns to the prehemorrhage risk level. Pathogenesis Varices in the distal esophagus and proximal stomach are a component of the collateral network that diverts high pressure portal venous flow through the left and right gastric veins and the short gastric veins to the azygous system. Less commonly, varices develop at other sites in the gastrointestinal tract but are less prone to rupture in those locations. Esophagogastric varices do not develop until portal pressure exceeds 12 mm. Hg and, once present, bleed in only one third to one half of patients. The pathogenesis of variceal rupture is incompletely understood but is most likely multifactorial. Polio and Groszmann 31 have put forth a unifying hypothesis of variceal rupture based on LaPlace's law. Although it has been observed that variceal size, magnitude of portal pressure, and thickness of the epithelium overlying the varix all significantly separate bleeders from nonbleeders, the overlap between groups is large when any one of these variables is considered independently. LaPlace's law states that variceal wall tension is directly related to transmural pressure and varix radius and inversely related to variceal wall thickness, thus combining all three of these variables. Because all of these parameters cannot be measured clinically, there are inherent inaccuracies in predicting which patients with varices may bleed. However, endoscopic classification schemes that consider size of varices and characteristics such as cherry-red spots and red wale markings, which are related to the thickness of the overlying epithelium, have improved the predictability of variceal hemorrhage. These prognostic indices are especially important when considering prophylactic therapy (treatment of varices that have not previously bled). Treatment Therapy for portal hypertension and variceal bleeding has evolved over the past 100 years. The many treatment modalities available suggest that no single therapy is entirely satisfactory for all patients or for all clinical situations. Nonoperative treatments are generally preferred for acutely bleeding patients since they are often high operative risks because of decompensated hepatic function. Therapies that are effective (a low rebleeding rate) and minimally alter hepatic physiology are optimal for long-term prevention of recurrent bleeding. Only treatments associated with minimal morbidity and mortality can be considered for prophylaxis, because many patients will be treated unnecessarily (only one third to one half of patients with varices eventually bleed). History of Treatment for Portal Hypertension Eck's main concerns were to determine if survival was possible after complete portal flow diversion and to develop a treatment for ascites. Probably the most important contribution to this field was made by Pavlov's group in 1893. These investigators perfected the technique of portacaval shunting and, after carefully observing 20 surviving dogs, described in detail the syndrome of “meat intoxication” or portosystemic encephalopathy, which they believed was due to intestinally absorbed cerebral toxins bypassing their site of metabolism in the liver. They also found from autopsy studies that dogs with encephalopathy had patent portacaval shunts and atrophic livers whereas animals with normal cerebral function and preserved hepatic structure had thrombosed shunts and maintenance of hepatic portal perfusion through collateral vessels. The modern era of treatment for variceal hemorrhage can be dated from 1945, when Blakemore, Lord, and Whipple introduced the portacaval and conventional splenorenal shunts into clinical practice. Although balloon tamponade and endoscopic sclerosis of varices were initially described in the 1930s, these were found to be only temporizing measures. During the ensuing 20 years, several varieties of nonselective shunts (complete portal decompression and portal flow diversion) were described and the portacaval shunt was evaluated in randomized, controlled trials. Motivated by the discouraging results of these trials, Warren, Zeppa, and Fomon introduced the concept of selective variceal decompression (distal splenorenal shunt) in 1967. 46 An initial wave of enthusiasm for the distal splenorenal shunt (partial portal flow diversion) was followed by several randomized trials, which produced inconsistent results. A report from Johnston and Rodgers in 1973 of a large series of patients successfully treated by endoscopic sclerotherapy led to a resurgence of interest in this treatment, which has now become the most widely applied therapy for bleeding varices. 20 Although pharmacotherapy was first used for acute hemorrhage in 1956, drug treatment for long-term prevention of initial or recurrent hemorrhage is a phenomenon of the 1980s. Improved immunosuppression (cyclosporine) and surgical techniques have led to the recent widespread application of hepatic transplantation for patients with end-stage liver disease. Finally, a nonoperative means of portal decompression (transjugular intrahepatic portosystemic shunt [TIPS]), first described by Rosch in animals in 1969, has been widely applied to the problem of variceal bleeding. Treatment of the Acute Bleeding Episode Because many patients with acute variceal bleeding have decompensated hepatic function secondary to either recent alcoholism or hypotension, they are high risks for emergency surgical intervention. 26 In addition, these individuals often have other complications of chronic liver disease such as encephalopathy, ascites, coagulopathy, and malnutrition. Therefore, emergency treatment should be nonoperative whenever possible. Endoscopic treatment (sclerosis or ligation), which has become the mainstay of nonoperative treatment of acute hemorrhage in most centers, controls bleeding in over 85% of patients, allowing an interval of medical management for improvement of hepatic function, resolution of ascites and encephalopathy, and enhancement of nutrition before definitive treatment for prevention of recurrent bleeding. Although pharmacotherapy and balloon tamponade still play an important role in emergency management in some patients (e.g., endoscopic treatment failures and those bleeding from gastric varices), these modalities are used less frequently than in the past. The place of the TIPS in managing acute variceal bleeding is evolving. Emergency surgical intervention may be the preferred approach in selected patients, especially those in whom less invasive methods fail to control bleeding or are not indicated. Resuscitation and Diagnosis. The highest priority in emergency management is restoration of circulating blood volume, which should be accomplished before upper gastrointestinal endoscopy. Although initial resuscitation is usually with isotonic crystalloid solutions, a minimum of six units of blood should be typed and crossmatched for most patients with variceal bleeding. Volume status is assessed by central venous pressure measurements, urinary output measured with a Foley catheter, and a Swan-Ganz pulmonary artery catheter if necessary. If the prothrombin time is prolonged more than 3 seconds, fresh frozen plasma should be a component of the resuscitation volume. Although moderate hypersplenism is a common accompaniment of portal hypertension, platelet transfusions are necessary only when the platelet count is less than 50,000 per cu. mm. Endoscopy to determine the cause of bleeding should be performed as soon as the patient is stabilized. If a bleeding esophageal varix is observed or suspected because of an overlying clot, sclerotherapy should be performed during the initial endoscopy if the expertise is available. Bleeding from gastric varices or from portal hypertensive gastropathy should be initially treated with pharmacotherapy. Because these lesions are often incompletely controlled by nonoperative means, such patients frequently require either insertion of a TIPS or early surgical intervention. Pharmacotherapy. Vasopressin, which is a potent splanchnic vasoconstrictor, has been the most commonly used drug in the acute setting and controls hemorrhage in approximately 50% of patients. Vasopressin is usually administered intravenously as a bolus dose of 20 units over 20 minutes and then as a continuous infusion of 0.4 units per minute. Because vasopressin also constricts systemic arterioles, it frequently causes hypertension, bradycardia, decreased cardiac output, and coronary vasoconstriction. Therefore, the use of this drug should be confined to the intensive care unit where the patient can be appropriately monitored. The adverse systemic effects of vasopressin can be effectively counteracted by simultaneous infusion of nitroglycerin or nitroprusside. The combination of vasopressin and nitroglycerin may also be more effective in controlling variceal hemorrhage than vasopressin alone. Recent randomized trials have shown that somatostatin and its analog octreotide are as efficacious as endoscopic treatment for control of acute variceal bleeding. These agents are also associated with fewer adverse side effects than vasopressin. Because of their ease of administration and effectiveness, these newer drugs may return pharmacotherapy to a more central role in the treatment of acute portal hypertensive bleeding, especially when endoscopic treatment is unlikely to be effective (failed chronic sclerotherapy, gastric varices, and portal hypertensive gastropathy). Somatostatin is administered as a 250-mg. intravenous bolus, followed by a continuous infusion of 250 mg. per hour for 2 to 4 days. Octreotide is given as an intravenous infusion of 25 to 50 mg. per hour for a similar length of time. Balloon Tamponade. The major advantages of variceal tamponade with the SengstakenBlakemore tube are immediate cessation of bleeding in more than 85% of patients and widespread availability of this device, including small community hospitals. Significant disadvantages of balloon tamponade are frequent recurrent hemorrhage after balloon deflation, considerable discomfort for the patient, and a high incidence of serious complications when the device is used by inexperienced personnel. The potentially lethal complications of esophageal perforation secondary to intraesophageal inflation of the gastric balloon, ischemic necrosis of the esophagus secondary to overinflation of the esophageal balloon, and aspiration can be avoided by using balloon tamponade only in an intensive care unit and adhering to a strict protocol. Because of the effectiveness of endoscopic treatment and pharmacotherapy for acute variceal bleeding, balloon tamponade is infrequently required. However, it may be lifesaving when exsanguinating hemorrhage prevents acute endoscopic treatment and in patients in whom sclerotherapy has failed and who do not respond to pharmacotherapy. Because balloon deflation is followed by a high rebleeding rate, definitive treatment such as endoscopic therapy, TIPS, or operation should be planned for most patients in whom the Sengstaken-Blakemore tube is used. Endoscopic Treatment. Endoscopic treatment (variceal sclerosis or ligation) is the most commonly used therapy for both management of the acute bleeding episode and prevention of recurrent hemorrhage. In early trials, variceal sclerosis was performed through a rigid esophagoscope with the patient under general anesthesia. However, most sclerotherapists now prefer to use a flexible endoscope because complications are fewer and general anesthesia can be avoided. Both intravariceal and paravariceal techniques of injection are used, and often these two techniques are purposefully or inadvertently combined. The most commonly used sclerosants in the United States are sodium morrhuate and sodium tetradecyl sulfate. A subsequent treatment session is planned for 4 to 6 days later. Additional endoscopic treatments depend on the effectiveness of the initial ones in controlling bleeding and whether endoscopic therapy has been selected as definitive treatment for that patient. Emergency sclerotherapy of esophageal varices has been highly effective with control of hemorrhage in over 85% of patients. However, this technique has been generally unsuccessful for bleeding gastric varices. Controlled trials have confirmed the effectiveness of endoscopic treatment in acutely bleeding patients. 26 Recent investigations have suggested that rubber band ligation of varices may be more effective and associated with fewer complications than sclerosis. Minor complications of sclerotherapy, including retrosternal chest pain, esophageal ulceration, and fever, occur commonly. More serious complications, which account for the 1% to 3% mortality rate of this procedure, are esophageal perforation, worsening of variceal hemorrhage, and aspiration pneumonitis. Failure of sclerotherapy should be declared when two sessions fail to control hemorrhage. Unless urgent surgery is performed in such patients, mortality exceeds 60%. Transjugular Intrahepatic Portosystemic Shunt. TIPS is a technique that accomplishes portal decompression without an operation. Because of the complexity of the procedure, an experienced interventional radiologist is required. Access is gained to a major intrahepatic portal venous branch through puncture through a hepatic vein. A parenchymal tract between hepatic and portal veins is then created with a balloon catheter and a 10-mm. expandable metal stent is inserted, thereby creating the shunt. In large series, the success rate of TIPS has been over 90%, but experience with this technique is limited in acutely bleeding patients, who generally make up only a small fraction of patients receiving TIPS. One evaluation of TIPS in the emergency setting revealed an in-hospital mortality of 56% and incomplete control of bleeding in 26% of patients. 11 At the present time, TIPS should not be recommended as initial therapy for acute variceal hemorrhage but should be used only after less invasive treatments such as endoscopic therapy and pharmacotherapy have failed to control bleeding. One clear indication for TIPS is as a short-term bridge to liver transplantation for patients in whom endoscopic treatment has failed. In addition to controlling bleeding, advantages in this situation are that the lower portal pressure may make the transplant operation easier and that the shunt is removed when the recipient liver is excised. Patients with advanced hepatic functional decompensation (Child's Class C), even when they are not transplant candidates, may be better served by TIPS than by an emergency operation when less invasive approaches fail to control bleeding. Hemodynamic studies suggest that TIPS is a nonselective shunt and several investigations have demonstrated a similar frequency of encephalopathy after TIPS as has been previously reported after nonselective shunts. Another disadvantage of the procedure is that shunt stenosis or occlusion develops in as many as 50% of patients within 1 year of TIPS insertion. 6 This situation can often be remedied by repeated angiographic intervention, however. Emergency Surgery. Although nonoperative therapies are effective for the majority of patients with acute variceal bleeding, emergency operation should be promptly done when less invasive measures fail to control hemorrhage or are not indicated. The most common situations requiring either urgent or emergency surgery are failure of acute endoscopic treatment, failure of long-term endoscopic therapy, and hemorrhage from gastric varices or portal hypertensive gastropathy. Selection of the appropriate emergency operation should mainly be guided by the experience of the surgeon. Esophageal transection with a stapling device is rapid and relatively simple, but rebleeding rates after this procedure are high and there is little evidence that operative mortality rates are less than after surgical portal decompression. The most commonly performed shunt operation in the emergency setting is the portacaval shunt because it rapidly and effectively decompresses the portal venous circulation. However, when the patient is not actively bleeding at the time of surgery and in those individuals in whom bleeding is temporarily controlled by pharmacotherapy or balloon tamponade, a more complex operation such as the distal splenorenal shunt may be appropriate. The major disadvantage of emergency surgery is that operative mortality rates exceed 25% in most reported series. Early postoperative mortality is usually related to the status of hepatic functional reserve rather than to the type of emergency operation selected. Prevention of Recurrent Hemorrhage Once a patient has bled from varices, the likelihood of a repeat episode exceeds 70%. Because most persons with variceal hemorrhage have chronic liver disease, the challenge of long-term management is both prevention of recurrent bleeding and maintenance of satisfactory hepatic function. Options available for definitive treatment include pharmacotherapy, chronic sclerotherapy, TIPS, three hemodynamic types of shunt operations (nonselective, selective, and partial), a variety of nonshunt procedures, and hepatic transplantation. Pharmacotherapy. Pharmacotherapy for the prevention of recurrent variceal bleeding was introduced in 1984 by Lebrec and co-workers, who reported that a dose of propranolol sufficient to decrease the heart rate by 25% resulted in a decreased frequency of recurrent hemorrhage and prolongation of survival in good-risk patients with alcoholic cirrhosis. Multiple subsequent investigations have shown inconsistent results. 24 Invasive hemodynamic monitoring of patients on propranolol has demonstrated minimal or no reduction of portal pressure in many individuals and no correlation between decrease in portal pressure and reduction in pulse rate, which has been the parameter used in most studies to assess therapeutic effect. Thus, two obstacles to effective treatment with propranolol are variability of response to the drug and the lack of an easily measured hemodynamic index to monitor therapy. A meta-analysis of the many controlled trials of nonselective beta-adrenergic blockade has shown it to decrease the likelihood of recurrent hemorrhage by about 20%. 24 Although multiple other drugs and combinations of agents have been tested, none has been demonstrated to be superior to nonselective beta-blockers. Long-term pharmacotherapy should be limited for use in compliant patients who are observed closely by their physicians. Although an attractive approach because of its noninvasiveness, pharmacotherapy is not yet a practical option for most patients who bleed from varices. Endoscopic Therapy. During the past 15 years, chronic endoscopic therapy has become the most common treatment for prevention of recurrent variceal hemorrhage. The increasing popularity of endoscopic treatment can be attributed to several factors: (1) disenchantment with shunt surgery among several gastroenterologists and surgeons; (2) endoscopic therapy is less invasive than surgery; (3) there are no adverse hemodynamic effects of endoscopic therapy; (4) endoscopic treatment can be administered by gastroenterologists to whom most patients are initially referred; and (5) several controlled trials have confirmed its therapeutic efficacy. The objective of chronic endoscopic therapy is to eradicate esophageal varices . Although the timing of repeat sessions varies from series to series, variceal eradication is usually successful in approximately two thirds of patients. Fewer treatment sessions are required to eradicate varices when variceal ligation rather than sclerosis is used. After eradication is achieved, diagnostic endoscopy should be performed at 6-month to 1-year intervals, because varices do recur and recurrent varices can bleed. Some investigators have noted an increased frequency of bleeding from gastric varices and portal hypertensive gastropathy after eradication of esophageal varices. Several controlled trials comparing chronic endoscopic therapy to conventional medical management have been completed. Although fewer patients receiving endoscopic treatment rebled than medically treated patients in all of the investigations, recurrent bleeding still occurred in approximately 50% of endoscopic therapy patients. Rebleeding is most frequent during the initial year and the rate decreases to approximately 15% per year thereafter. Although a single episode of recurrent hemorrhage does not signify failure of therapy, uncontrolled hemorrhage, multiple major episodes of rebleeding, and hemorrhage from gastric varices and portal hypertensive gastropathy all require that endoscopic therapy be abandoned and another treatment modality substituted. Endoscopic treatment failure secondary to rebleeding occurs in as many as one third of patients. Thus, chronic endoscopic therapy is a rational, initial treatment for many patients who bleed from esophageal varices, but subsequent treatment with TIPS, a shunt procedure, nonshunt operation, or hepatic transplantation should be anticipated for a significant percentage of patients. Because of its relatively high failure rate, a course of chronic endoscopic therapy should not be undertaken for noncompliant patients and those living a long distance from advanced medical care. PORTOSYSTEMIC ENCEPHALOPATHY Portosystemic encephalopathy is a psychoneurologic syndrome that may have a variety of manifestations, including alterations in the level of consciousness, intellectual deterioration, personality changes, and neurologic findings such as the flapping tremor, asterixis. 7 Although encephalopathy may sometimes develop spontaneously in patients with chronic liver disease, it more often occurs after portosystemic shunt procedures. It is particularly common (20% to 40% of patients) in individuals who undergo nonselective shunts. Although the pathogenesis of encephalopathy is uncertain, most theories are based on circulating cerebral toxins that are intestinally absorbed and bypass the liver by means of shunts or fail to be inactivated by the liver's decreased metabolic capacity. Purported cerebral toxins include ammonia, mercaptans, and gamma-aminobutyric acid. The false neurotransmitter hypothesis, based on the high ratio of aromatic to branched-chain amino acids present in the blood of patients with chronic liver disease, has also been proposed to explain the psychoneurologic disturbances observed. Encephalopathy develops spontaneously in less than 10% of patients. More commonly, one or more of the following precipitating factors induce the syndrome: gastrointestinal hemorrhage, excessive diuresis, azotemia, constipation, sedatives, infection, and excess dietary protein. Most episodes of encephalopathy can be successfully treated by identifying and then eliminating the precipitating factors responsible. Dietary protein should be restricted, infections should be treated, all sedatives should be discontinued, and intestinal catharsis to remove blood within the gastrointestinal tract should be accomplished. Stool softeners and dietary protein restriction should be prescribed for patients who have chronic encephalopathy. Pharmacologic treatment of encephalopathy is indicated for individuals with chronic, intermittent symptoms and for those with persistent, acute psychoneurologic disturbances despite elimination of precipitating factors. The only drugs with proven effectiveness are neomycin, a poorly absorbed antibiotic that suppresses urease-containing bacteria, and lactulose, a nonabsorbable disaccharide that acidifies colonic contents and also has a cathartic effect. Acute episodes of encephalopathy can be treated equally effectively by neomycin and lactulose. Neomycin is orally administered in a dose of 1.5 gm. every 6 hours, whereas in the acute setting lactulose should be given in a dose of 30 gm. every 1 or 2 hours until a cathartic effect is noted. The patient should then be maintained with 20 to 30 gm. of lactulose two to four times a day or as needed to result in two soft bowel movements daily. Neomycin should not be used for treatment of chronic encephalopathy because nephrotoxicity or ototoxicity may develop. Lactulose combined with mild protein restriction (60 to 80 gm. per day) is the preferred treatment for chronic, intermittent encephalopathy. Enteral or parenteral administration of nutritional formulas enriched in branched-chain amino acids has been suggested as the treatment of both acute and chronic encephalopathy. These solutions have improved mental status in patients in some controlled trials. Surgery plays a minor role in the management of encephalopathy. Cerebral function has been improved in some patients with chronic encephalopathy after interruption of a completely diverting portosystemic shunt (nonselective shunt). Likewise, in isolated cases, occlusion of a major portosystemic collateral vessel such as the coronary vein has reversed encephalopathy after the distal splenorenal shunt. Although both total colectomy and colonic exclusion have resolved encephalopathy in some patients, the significant morbidity and mortality rates after these operations in patients with decompensated hepatic disease have prevented their widespread use. ASCITES Ascites is another complication of portal hypertension that rarely requires surgical treatment. 1 Portal hypertensive ascites is initiated by altered hepatic and splanchnic hemodynamics, which cause transudation of fluid into the interstitial space. When the rate of interstitial fluid formation exceeds lymph drainage capacity, ascites accumulates. This pathophysiologic process results in an intravascular volume deficit, which initiates compensatory mechanisms such as aldosterone secretion to restore plasma volume. Both the liver and intestines are important sites of ascites formation, and clinically detectable ascites is rare in patients with extrahepatic portal hypertension. Medical management resolves ascites in approximately 95% of patients. 1 Dietary salt restriction and diuretic therapy are the mainstays of medical treatment. A rational first-line diuretic is spironolactone because secondary hyperaldosteronism is present in most patients. A combination of salt restriction (20 to 30 mEq. per day) and spironolactone in a dose of 100 to 400 mg. per day results in effective diuresis in approximately two thirds of patients. 1 If this regimen fails to mobilize ascites, a more potent diuretic such as hydrochlorothiazide or furosemide should be added. Recent studies have shown that intermittent, large-volume paracentesis either with or without intravenous infusion of colloid is also effective. 1 Contrary to earlier beliefs, large-volume paracentesis in a stable patient does not markedly alter systemic hemodynamics or renal function. Surgical therapy should be reserved for the unusual patient with medically intractable ascites. The safest and most effective surgical procedure is the peritoneovenous shunt. However, controlled trials have shown that this relatively simple operation is followed by significant morbidity and that survival is not prolonged when compared with medical management. Although side-to-side portosystemic shunts are also effective in relieving ascites, these operations should not be used for ascitic patients unless they have bled from esophagogastric varices. TIPS, a nonoperative side-to-side portosystemic shunt, has also been effectively used to relieve medically intractable ascites. However, because of the high long-term failure rate of TIPS and the adverse consequences of total portal diversion secondary to this procedure (i.e., encephalopathy and accelerated hepatic failure), its use should be limited to highly selected patients. PERITONEOVENOUS SHUNTS FOR INTRACTABLE ASCITES Ascites is the pathologic accumulation of fluid in the peritoneal cavity. It may be part of a generalized third-space fluid loss in conditions such as chronic renal disease, congestive heart failure, or massive fluid overload, but it is more often related to intra-abdominal factors that cause formation of peritoneal fluid at a more rapid rate than it can be absorbed. Of these conditions, chronic liver disease is by far the most common. PATHOPHYSIOLOGY OF ASCITES FORMATION IN CIRRHOSIS In cirrhosis with portal hypertension, the accumulation of ascites is caused by abnormal renal function that is characterized by sodium and water retention. 1 The degree of renal dysfunction ranges from minimal in compensated cirrhosis without ascites with only a mild abnormality of sodium homeostasis (e.g., inability to excrete a sodium load) to advanced dysfunction in decompensated cirrhosis with marked sodium retention and progressive ascites. These patients have a reduction in renal blood flow with decreased glomerular filtration rate from intrarenal shunting of blood to medullary nephrons in spite of normal or increased circulating blood volume and cardiac output. Associated with these renal and hemodynamic changes are increases in plasma renin, angiotensin, and aldosterone levels. These factors contribute to sodium and water retention with low urinary volume and sodium concentration. The end stage of this spectrum is a functional form of renal failure with progressive oliguria, rising creatinine, and intense sodium retention, termed hepatorenal syndrome. 2 Studies of the mechanism of ascites formation have enhanced understanding of many aspects of this functional renal disorder that accompanies postsinusoidal portal hypertension; however, there is no single theory that unifies the many pathophysiologic disturbances that have been identified. The classic underfill theory focuses on portal hypertension and hypoalbuminemia as the initiating factors in ascites formation. The fibrosis and nodular regeneration that occur in the cirrhotic liver increase the resistance to portal blood flow, which increases the pressure in the portal and splanchnic veins. This increased hydrostatic pressure promotes the movement of fluid from the intravascular to the extravascular compartment. Although some of this increased interstitial fluid can be taken up by mesenteric lymphatics (producing increased flow in the thoracic duct), fluid production exceeds the capacity of lymphatic absorption and ascites is produced off the surfaces of the liver, bowel, and mesentery. The hypoalbuminemia associated with liver disease may contribute to the formation of ascites through a decrease in oncotic pressure in the postcapillary venule. The underfill theory postulates that portal hypertension and hypoalbuminemia produce an imbalance of Starling forces across the portal vasculature with the loss of intravascular volume as ascites. Together with sequestration of blood in the splanchnic bed (caused by portal hypertension), this third-space loss of ascitic fluid produces a decrease in the effective circulating volume. This accounts for the secondary decreases in renal blood flow and glomerular filtration rate and stimulation of the renin-angiotensin-aldosterone system, which all contribute to sodium and water retention. This retention of sodium and water further aggravates the ascites, and the process becomes cyclical. The underfill theory, however, which focuses on portal hypertension as the primary etiologic factor, does not explain why in conditions of presinusoidal portal hypertension, including schistosomiasis and portal vein thrombosis, ascites was uncommon whereas in postsinusoidal obstruction, including acute right-sided heart failure and Budd-Chiari syndrome, ascites was characteristic. It has now become clear that renal sodium and water retention with plasma volume expansion occurs before the development of ascites in man. This is the basis of the overflow theory originally proposed by Lieberman and associates in 1970, 3 which postulates a primary abnormality of renal function with progressive sodium and water retention in patients who have postsinusoidal portal hypertension. The cause of this lesion has yet to be defined. 4 It may be initiated by intrahepatic pressure receptors and may be neural or hormonally mediated. Abnormalities of atrial natriuretic factor responsiveness, impaired intrarenal prostaglandin production, sympathetic nervous system overactivity and unresponsiveness, and increased production of nitric oxide 5 may contribute to the progressive renal sodium retention. Sodium and water retention lead to expansion of the plasma volume. The alteration in Starling forces identified in the underfill theory account for the subsequent accumulation of the retained sodium and water as ascites in the abdominal cavity. The response to peritoneovenous shunting has been used to study the pathogenesis of ascites. Acutely, it decompresses the peritoneal cavity and, by establishing a sustained volume expansion, it has identified the role of hemodynamics, renin and aldosterone, vasopressin, prostaglandins, catecholamines, 10 and atrial natriuretic factor 4 in the etiology of sodium and water retention. Although the overflow theory explains many of the observations early in the pathogenesis of ascites, it appears insufficient to explain the spectrum of renal dysfunction, particularly in decompensated cirrhosis, where the increased sympathetic nervous system activity, relative arterial hypotension, and elevated renin-angiotensin-aldosterone activity are more consistent with circulatory underfilling than volume expansion. The most recent theory—the peripheral arterial vasodilatation hypothesis proposed by Schrier and colleagues 11 in 1988—suggests that the integrity of the arterial circulation, not the total plasma volume, is the major determinant of renal sodium and water handling. It proposes that the progressive arterial vasodilatation accompanies the stages of cirrhosis (from compensated to decompensated to hepatorenal syndrome). The major site of this vasodilatation may be the splanchnic arteries; however, the etiology of this vasodilatation remains unclear. Many factors, including opening of pre-existing shunts, increased nitric synthase activity, vasodilating prostaglandins, and others have been proposed. Arterial vasodilatation is accompanied by activation of the neurohumoral responses to arterial underfilling, which causes sodium and water retention and leads to plasma volume expansion. It is postulated that much of this expansion occurs in the venous compartment, which is estimated to comprise over 85% of the circulating volume, and that arterial refilling is not achieved. Sodium and water retention therefore persists. Whether this hypothesis can account for the events that initiate sodium retention or whether the primary hepatorenal dysfunction postulated by the overflow hypothesis can induce the onset of ascites without arterial vasodilatation remains to be determined. CLINICAL MANIFESTATIONS OF ASCITES IN CHRONIC LIVER DISEASE Although the development of ascites is usually chronic, ascitic fluid may accumulate rapidly in the presence of a hepatoma, hepatic vein thrombosis, or acute hepatitis superimposed on chronic cirrhosis. Transient ascites may develop after a laparotomy or from aggressive resuscitation of patients with variceal bleeding with large volumes of fluid. The presence of ascites in the patient with chronic liver disease usually indicates an advanced degree of liver dysfunction and is associated with a poor prognosis. One-year survival figures of less than 50% have been reported for patients with resistant ascites, 12 a prognosis that is similar to that of patients who have bled from esophageal varices. In well-compensated cirrhotic patients, a small amount of ascites may go unnoticed, other than for a slight increase in abdominal girth, and requires no treatment. Severe ascites, however, may become disabling, with significant interference in activities of daily living. There may be pleural effusions and atelectasis with shortness of breath, swelling of the legs, and the development of umbilical and inguinal hernias. Patients with ascites are at risk for developing spontaneous bacterial peritonitis, which carries a 70% mortality rate. Abdominal distention may produce anorexia, which when combined with dietary restrictions for encephalopathy causes protein-calorie malnutrition with progressive muscle wasting. MEDICAL TREATMENT OF ASCITES When ascites becomes symptomatic, treatment is directed toward the reduction, but not necessarily the complete elimination of ascites. Some patients can be managed with dietary restriction of 1 gm. sodium and 1 liter of fluid per day. If the ascites is more resistant, diuretics may be added, beginning with an aldosterone antagonist such as spironolactone or amiloride. If this is insufficient, a loop diuretic, such as furosemide or a thiazide, should be added. In general, loop diuretics should not be the sole therapeutic agents. Diuretics should be added cautiously, and renal function must be closely monitored. In general, any peripheral edema responds to diuretics before ascites. Once dependent edema has resolved, diuretic therapy should aim to mobilize no more than 1 liter of fluid or 1 kg. of body weight per day. Serious complications can result from the chronic and overly aggressive use of diuretics in cirrhotic patients, particularly when combined with intermittent therapeutic paracentesis. These include hyponatremia, hypokalemia, metabolic alkalosis, hypocalcemia, and hypomagnesemia. When these diuretics are used, muscle cramps, particularly in the calves, are common and difficult to manage. Excessive diuresis can aggravate hypovolemia and produce prerenal failure with oliguria and acute tubular necrosis or may precipitate the hepatorenal syndrome. Therapeutic paracentesis can be effective in removing all ascites and may be necessary in patients with tense ascites with shortness of breath from the increased intra-abdominal pressure and elevated diaphragm. Despite previous concerns that after large-volume paracentesis (more than 5 liters) the reaccumulation of ascites might reduce intravascular volume and precipitate hypotension and/or renal failure, a number of carefully designed trials have demonstrated the safety of carefully supervised, repeated paracenteses with 5% albumin infusion 13 or single total paracentesis with albumin infusion. 14 These studies demonstrated that, compared with diuretic therapy alone, paracentesis with albumin infusion is more effective in eliminating ascites, does not cause significant changes in hemodynamics or hepatic or renal function, was associated with a lower incidence of electrolyte abnormalities, and had a shorter hospitalization with similar rates of readmission and survival. A subsequent trial of large-volume paracentesis without albumin infusion, however, was associated with significant impairment of renal function, 15 emphasizing the importance of colloid infusion after large volume paracentesis and close monitoring of the patient. Smaller, repeated paracenteses without colloid infusion appear to be safe. 13 In a randomized trial comparing paracentesis and albumin infusion against the LeVeen shunt, peritoneovenous shunting controlled the ascites better in the long term; however, total hospitalization and overall survival were the same. 16 Intermittent largevolume paracentesis with albumin replacement (performed on an outpatient basis) with judicious use of sodium restriction and diuretics and close monitoring of renal function has become a common strategy for the long-term management of diuretic-resistant ascites. This technique is also valuable in the cirrhotic patient who develops acute ascites after portosystemic shunt to avoid an ascitic leak through the wound. SURGICAL TREATMENT OF ASCITES The majority of patients with ascites respond to diet and diuretic therapy. In those with persistent ascites and adequate hepatic function, surgical intervention may be an alternative to long-term, repeated large-volume paracentesis. Surgical procedures for intractable ascites have been directed toward either decreasing production of ascites or increasing its resorption. Portosystemic Shunting. Physiologic side-to-side portosystemic shunts (side-to-side portacaval, mesocaval, or proximal splenorenal anastomoses) lower pressure in both the splanchnic veins and the intrahepatic portal system, which theoretically should decrease the rate of ascites formation. There is substantial evidence from animal models and in patients with the Budd-Chiari syndrome to support the use of side-to-side shunting when both bleeding and uncontrolled ascites require surgical treatment. 17 In a study of patients undergoing a portosystemic shunt procedure for bleeding esophageal varices, compared with patients managed with injection sclerotherapy or esophageal transection, the probability of developing ascites was lower (15% vs. 73%) and the incidence of both spontaneous bacterial peritonitis and hepatorenal syndrome was lower (2% to 4% vs. 21% for each) in the shunted group. Side-to-side shunting has been used for intractable ascites alone, but because of the mortality and the incidence of postshunt encephalopathy, the risk of portosystemic shunting for ascites alone has been considered prohibitive. More recent experience with portosystemic shunting for ascites alone in carefully selected patients has confirmed its effectiveness in controlling ascites, but the 50% incidence of encephalopathy is a strong argument against its general acceptance. Transjugular Intrahepatic Portosystemic Shunt. The transjugular intrahepatic portosystemic shunt was first described in man in 1982 as therapy for bleeding varices. 20 With the addition of an expandable stent to maintain patency, its ability to significantly reduce portal pressure has been applied more widely. 21 It has become attractive as a bridge to liver transplantation in patients whose bleeding varices have not been well controlled with sclerotherapy. 22 Hemodynamically, it functions as a type of side-to-side portacaval shunt. It may be expected therefore that this shunt should be effective in controlling ascites. Preliminary reports of small numbers of patients who had ascites at the time of the shunt procedure have suggested a response rate of 50% to 67%. 20, 24 The complications of shunt occlusion (over 30%) and hepatic encephalopathy (over 20%) may limit the usefulness of this new technology as primary therapy for ascites. Peritoneovenous Shunting. Peritoneovenous shunting is based on an extension of the principles of paracentesis with reinfusion studies and involves the implantation of a prosthetic conduit with a one-way valve between the peritoneal cavity and the intrathoracic vascular compartment. The shunt receiving the widest use and most careful study is that devised by LeVeen and first reported in 1974 . 25 It consists of a pressure-activated oneway valve with peritoneal and venous Silastic catheters. The pressure gradient between positive intraperitoneal and negative intrathoracic pressures promotes fluid flow from the abdomen to the intrathoracic vascular space. Other valves, such as the Denver shunt , 26 have been developed as alternatives to the LeVeen valve and incorporate features that allow active pumping of fluid from the peritoneal cavity to the venous compartment. One randomized trial in 21 cirrhotic patients has suggested that the LeVeen valve has a superior patency rate of 40% at 2 years compared with the Denver shunt; however, the survival was similar in the two groups. 27 Indications. The most common indication for a peritoneovenous shunt is chronic intractable ascites due to cirrhosis. Up to 10% of patients with significant ascites become refractory to management with diet and diuretics with or without repeated large-volume paracentesis and colloid reinfusion. Intractable ascites has been associated with progressive muscle loss, and repeated paracentesis may be complicated by intra-abdominal bleeding, bacterial infection, and loculation of ascites. In some patients, a strategy of repeated paracentesis may not be logistically possible and others may require repeated hospital admissions for their ascites. The peritoneovenous shunt may be effective in achieving better long-term control. 16 The results of peritoneovenous shunting vary depending on the severity of the renal and hepatocellular dysfunction of the patient being shunted. The shunt should not be used in patients who can be managed with diet and diuretics but should be reserved for those who fail closely supervised medical management. The peritoneovenous shunt has been used in the treatment of the hepatorenal syndrome. This form of functional renal failure must be distinguished from prerenal failure produced by diuretic- or paracentesis-induced central blood volume constriction with acute tubular necrosis. The peritoneovenous shunt has been reported to reverse the hepatorenal syndrome, but because of advanced liver disease, these patients are a very high risk for the procedure and postoperative complications. Postoperative ascites may develop in the cirrhotic patient after any abdominal operation, but especially after portosystemic shunting procedures. This may be secondary to aggressive intravenous therapy in the perioperative period combined with the division of periportal or retroperitoneal lymphatics at the time of the procedure. Repeated large-volume paracentesis with colloid infusion is successful in most patients. If the postoperative ascites is severe and reaccumulates rapidly, an early peritoneovenous shunt is usually effective. Contraindications. Peritoneovenous shunting is contraindicated in any patient with infected peritoneal fluid or other source of sepsis, acute viral or alcoholic hepatitis, end-stage liver disease, or uncorrectable coagulopathy. A serum bilirubin value of more than three times the upper limit of normal has generally been considered a contraindication. The presence of any one of the following risk factors has been reported to be associated with a 50% mortality within the first postoperative month and should be considered a relative contraindication 31: (1) an episode of gastrointestinal bleeding or peritonitis within the previous month; (2) hepatic encephalopathy greater than Grade 1; (3) complications of alcoholism such as pancreatitis, cardiomyopathy, or neuropathy; (4) an uncomplicated hernia; (5) severe malnutrition; (6) a prothrombin time prolonged more than 4 seconds; or (7) a serum creatinine value more than 2.3 times the upper limit of normal. Patients who have had a previous variceal hemorrhage remain at risk for rebleeding after shunting because of the transient increase in portal pressure and the coagulopathy that accompanies the procedure. If the esophageal varices have not been obliterated by repeated injection sclerotherapy, or there has been recent bleeding, patients may be better treated by a side-toside portosystemic shunt. Procedure. A diagnostic paracentesis is performed to exclude infection (negative culture and a cell count less than 250 × 10 6/L.) or malignancy. Liver biopsy (transjugular) and detailed liver tests including coagulation studies are performed. Perioperatively, prophylactic antibiotics, such as a cephalosporin and an aminoglycoside, should be administered to cover both skin and gastrointestinal tract organisms. The shunt may be inserted with the patient under local or general anesthesia . The LeVeen valve is placed in the abdominal wall deep to the rectus muscle. A pumpable valve (e.g., Denver shunt) is placed over the chest wall or lower end of the sternum. The peritoneal end lies freely in the ascites. The venous limb is tunneled subcutaneously and may be inserted into the superior vena cava through either the internal jugular or the subclavian vein. During the operation, the tip is directed under radiologic control to lie just below the junction of the superior vena cava and the right atrium. A tip that is left too high in the superior vena cava or innominate vein will predispose to catheter blockage and venous thrombosis. Meticulous hemostasis is important to prevent the occurrence of hematoma and ecchymosis. The incidence of postoperative coagulopathy may be reduced by draining all of the ascitic fluid and replacing it with body-temperature Ringer's lactate. Some surgeons discard most of the ascitic fluid at surgery to reduce the incidence of pulmonary edema. Prophylactic antibiotics are continued for up to 48 hours. Inspiratory exercises are used to decrease intrathoracic pressure and increase the gradient across the valve and promote ascites flow. Small doses of intravenous furosemide may be necessary to maintain the urine output at greater than 60 ml. per minute. Body weight, abdominal girth, and the hematocrit, serum electrolytes, and coagulation status are monitored closely. Results. With reinfusion of the ascitic fluid there is an increase in the total circulating blood volume, a hemodilutional fall in hematocrit, an increase in the cardiac output, and a decrease in the systemic vascular resistance. There is an early rise in renal blood flow and glomerular filtration rate and an associated decrease in renin and aldosterone levels. In some patients, spontaneous natriuresis and diuresis occur; however, most patients require small doses of furosemide to produce and maintain the diuresis and natriuresis that are characteristic of a functioning shunt and that lead to progressive loss of ascites, abdominal girth, and weight. Early postoperative complications occur in up to 80% of patients and include infection, leak of ascitic fluid, variceal hemorrhage, pulmonary edema, coagulation disorders, shunt migration or blockage, and cardiac arrhythmias. All patients develop a degree of coagulopathy with prolongation of the prothrombin and partial thromboplastin times and a fall in platelet count, and up to 20% may develop clinical manifestations of disseminated intravascular coagulation. The degree of coagulopathy and the incidence of disseminated intravascular coagulation have been reduced by discarding most of the ascites intraoperatively, with or without replacement with saline or Ringer's lactate. Clinical disseminated intravascular coagulation is treated by temporarily ligating the shunt and providing fresh frozen plasma, cryoprecipitate, and platelets. Epsilon-aminocaproic acid may also be useful in this setting. The coagulation defect is usually self-limited, resolving after a few days. Patients undergoing peritoneovenous shunting are a high risk group for infectious complications from malnutrition and reduced cell-mediated immunity. Previous episodes of spontaneous bacterial peritonitis increase the risk of postoperative shunt infection. When it occurs, shunt infection is a serious complication that always requires shunt removal and systemic antibiotic therapy. Blockage of the shunt may occur by omentum migration into the tubing, kinking, thrombus formation at the tip of the venous limb, or the collection of fibrin and other peritoneal debris within the valve. The diagnosis of shunt occlusion can be made with either an intraperitoneal injection of technetium-99m sulfur colloid and scanning of the chest 37 or a direct percutaneous injection of radiopaque contrast medium into the venous limb. With the latter technique, a more precise identification of point of occlusion (valve, venous tubing, or thrombus) can be made. In the event of a blocked valve with a patent venous limb, it is usually possible to replace only the valve and peritoneal limb. The operative mortality ranges up to 30%. The major causes of early death are infection and complications of the underlying liver disease, including hepatic failure and variceal hemorrhage. Variations in operative mortality and survival reported reflect the severity of the liver disease and renal dysfunction in the groups of patients being selected for shunting procedures. With careful patient selection and improved perioperative care, operative mortality may be less than 5%. The renal and hemodynamic changes persist in the late postoperative period. Most patients have normal levels of renin and aldosterone and persistent improvements in creatinine clearance and sodium and water excretion. However, many continue to retain sodium when challenged with a high-sodium diet, and most require small doses of diuretics even with a functioning shunt. Others become free of ascites without diet or diuretic therapy. In some patients, ascites remains under control with diuretics, even if the shunt becomes blocked. With the control of ascites, many patients demonstrate an improvement in nutritional status with an increase in serum albumin and transferrin levels, an improvement in cell-mediated immunity, and an increase in lean body mass with an obvious gain in muscle bulk. These improvements likely result from an improved well-being and increase in appetite and dietary intake. Late complications occur in a significant proportion of surviving patients and include shunt blockage, infection, superior vena caval thrombosis, and obstruction of the small bowel. The shunt patency rate of 5-year survivors has been reported to be as low as 40%, and the incidence of thrombosis of the superior vena cava has been over 25%. 40 With improved patient selection and perioperative technique, the shunt occlusion rate may be reduced to 12% at 2 years. 30 Most of the late postoperative deaths are due to bleeding esophageal varices and hepatic failure. Peritoneovenous shunting does not prolong life. One study comparing the peritoneovenous shunt with diuretic therapy in 28 patients refractory to sodium restriction alone suggested improved survival with the shunt. 43 However, two subsequent multicenter, randomized studies of 57 patients in France 34 and 299 men in the United States 31 showed no difference between diet and diuretic therapy and peritoneovenous shunting. In both groups, the early mortality (30 days) ranged from 20% to 50%, depending on the severity of the liver disease, and the 1-year survival ranged from 25% to 79%. These authors identified a modest benefit in favor of the shunt, which was limited to control of ascites predominantly in the first month. A subsequent prospective, randomized trial of LeVeen shunting versus repeated paracentesis with albumin infusion in 89 patients in Spain demonstrated equivalence between the two therapies. The ascites response rate was the same. Although the initial hospitalization was longer for the shunted patients, and the readmission rate was higher with paracentesis, total hospitalization was the same. The 3-year survival was the same (approximately 33%). In other uncontrolled series, the survival curves after insertion of the peritoneovenous shunt have been similar to older studies of patients who developed ascites before the introduction of the LeVeen valve, with a 50% to 75% 1-year survival and 30% to 40% survival at 2 years. A more recent report has suggested that with careful patient selection and improved perioperative technique, the 2-year survival after shunting may be improved to 55%. In cirrhotic patients with intractable ascites, peritoneovenous shunting should be considered to be only a palliative procedure. Although the operation is appealing because of its apparent technical simplicity and the early postoperative diuresis, the postoperative complication rate is high. Long-term survival appears to be determined mainly by the natural history of the liver disease. A review in 1990 concluded that the patient undergoing peritoneovenous shunting may be expected to have an 18% perioperative mortality, a 46% survival rate at 21 months, and control of ascites in 59% of those who survive more than 18 months. 41 The equivalent efficacy of either diuretic therapy or repeated paracentesis compared with shunting has led to a significant reduction in the use of the peritoneovenous shunt. Patients must be selected carefully. In a patient who is or may be a candidate for liver transplantation, peritoneovenous shunting should be avoided—it does not alter the course of the underlying liver disease, the procedure has a significant morbidity and mortality, and the occurrence of thrombosis of the superior vena cava poses a significant relative contraindication to the transplant operation. Two groups of patients appear to be the best candidates for peritoneovenous shunting: (1) those in whom uncontrollable ascites develops immediately after abdominal operations and (2) those whose chronic, intractable ascites is out of keeping with the other complications of portal hypertension and hepatocellular dysfunction. PERITONEOVENOUS SHUNT FOR MALIGNANT ASCITES Malignant ascites occurs most commonly with intraperitoneal spread of ovarian, colonic, or breast cancer caused by increased fluid production (from the tumor or peritoneal surface inflammation) and reduced reabsorption (from lymphatic obstruction). The majority of patients obtain palliation from a combination of paracentesis and chemotherapy and/or radiation therapy. In selected patients who are unresponsive to this treatment and who do not have loculated fluid, peritoneovenous shunting may be indicated and the ascites may be controlled in approximately two thirds of patients. In a series of 42 patients with malignant ascites treated with a peritoneovenous shunt, compared with treated with paracentesis followed by intraperitoneal doxorubicin, the ascites was controlled by the shunt in 64%. The median survival from the time of diagnosis of ascites was 137 days in the shunted group and 91 days in those not shunted. Survival was best for those with breast cancer. There was no difference in total hospitalization or measurements of quality of life between the two groups. 45 The equivalence of paracentesis with peritoneovenous shunting suggests a limited role for surgery in these patients with limited life expectancy. Complications are similar to those in the cirrhotic population, and the operative mortality is similar. Shunt occlusion is more frequent, especially in patients with bloody or viscid ascitic fluid, high protein content (greater than 4.5 gm. per liter), or positive cytology. These factors are therefore relative contraindications to its use. Tumor embolism occurs in approximately 5% of patients and occasionally produces acute respiratory failure and death. In spite of these complications, 60% to 75% of patients obtain useful palliation, with the best results occurring in patients with carcinoma of the ovary or breast. In general, the median reported survival, however, is only 3 months. VIRAL HEPATITIS AND THE SURGEON Blumberg's discovery of the Australia antigen in 1965 and the technological advances of the past three decades have led to a virtual explosion of new information about viral hepatitis. Although itself not a surgical disease, viral hepatitis is an important concern to both the surgical patient and the surgeon. In this section the basic concepts about the viral hepatitides are summarized, focusing particularly on those viruses most relevant to the practicing surgeon. Excellent texts, reviews, monographs, and papers are available and should be consulted for more detailed information. HISTORY DEFINITION Viral hepatitis is an infection of the liver caused by one of at least six groups of viruses: hepatitis A virus (HAV) (known formerly as causing infectious hepatitis); hepatitis B virus (HBV) (known formerly as causing serum hepatitis); hepatitis C virus (HCV), the recently recognized virus that is associated with parenterally transmitted non-A, non-B hepatitis (PT-NANB); hepatitis D virus (HDV), known also as the agent that causes delta-associated hepatitis; hepatitis E virus (HEV), the virus responsible for enterically transmitted non-A, non-B hepatitis (ET-NANB); and two new viruses, designated hepatitis F and G (HFV and HGV), that cause clinical disease but are distinct from each other and from hepatitis A–E. ETIOLOGY Nomenclature. Familiarity with the terms that describe the hepatitis virus antigens and antibodies is essential to the understanding of this group of diseases. No antigens common to two or more viral agents have been identified. Within each class of virus, no differences in the virulence or the nature of the ultimate disease have been found between subtypes or strains, although there is now a description of viral variants that seem to modify the immune response and therefore, potentially, the clinical consequences. 16 PATHOGENESIS The pathogenesis of viral hepatitis is incompletely understood, but two theories have been proposed. One theory suggests direct cytopathogenicity of liver cells by virus, and the other proposes humoral and cell-mediated immunopathogenetic mechanisms for all types of viral hepatitis. It is possible that both mechanisms occur. The extrahepatic manifestations of HBV, HCV, and HDV infections are thought to be due to the localization of antigen-antibody complexes in the affected tissue (synovium, arteries, kidneys, and skin). EPIDEMIOLOGY To understand viral hepatitis, it is important to consider the trends in occurrence, risk factors, and modes of transmission. In 1993, the Centers for Disease Control and Prevention (CDC) received reports of 24,238 cases of infection with HAV, 13,361 cases of infection with HBV, and 4786 cases of NANB infection. From these figures they project that in the United States 300,000 cases of HBV and 5000 deaths yearly are due to viralinduced cirrhosis and hepatocellular carcinoma. HAV infection increased in the late 1980s but is now on the decline. It still, however, accounts for 56% of all reported cases of hepatitis. HBV infection cases increased progressively through the mid 1980s and then steadily decreased, now accounting for 30% of the total. Although the number of reported cases of HCV infection is relatively low, it is the most common cause of posttransfusion hepatitis. The other hepatitis viruses are either rarely recognized or rarely reported in the United States. VIRAL TRANSMISSION Transmission of HBV and HCV through transfused blood is facilitated by the ability of these viruses to exist in an infectious form in the serum of otherwise normal blood donors, making the period of infectivity potentially very long. HDV, a defective virus that requires HBV for replication, may also persist chronically in asymptomatic individuals, although this has been more difficult to establish. Certainly HAV and HEV, and likely the two new agents as well, do not seem to constitute any special risk for the surgeon and will not be discussed further here in any detail. Blood and blood products from different sources have a greater or lesser risk of possessing infectious virus. Most whole blood and its derivatives that are not prepared by pooling of units have a relatively smaller risk of inducing infection with HBV. This is largely due to the infrequency of viral carriage in the normal adult population, the exclusion of potentially infected donors defined by questionnaire, and, perhaps most important, the routine testing of donor blood for hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg), HCV antibody, and serum alanine aminotransferase, positive results for which eliminate these units for use. Such procedures are especially important when the donor pool may include individuals at risk for HBV, HCV, and HDV infections, such as intravenous drug users and those who engage in high-risk sexual practices. The prevalence of one or another form of hepatitis in these groups approaches 100%. Preparations generally considered to be safe include serum albumin, thrombin, profibrinolysin, fibrinolysin, immune serum globulin, and all hyperimmune globulins, although one report identified contaminated intravenous immune globulin as the source of a sizable epidemic of HCV infection in Scandinavia. 11 The blood and blood products that pose a higher risk are those from commercial as opposed to volunteer donors and from pools of plasma and clotting factors. 11, 44 Washed and frozen human blood cells are not reliably virus free. Other predictable means of parenteral exposure include accidental needlestick or accidental injury, such as might occur during a surgical procedure. It is this occupational risk that represents a continuous threat over the period of a surgical career and constitutes the basis for individual protection, including scrupulous surgical technique and HBV vaccine. There is strong evidence that certainly HAV and HEV—and probably also HBV and HCV—may be transmitted by close, personal contact. Sexual transmission is one such route for HAV and HBV and possibly for HCV. It is currently not possible to quantify the frequency of nonparenteral transmission in nonepidemic circumstances, but cases of hepatitis B and C occur without a prior known opportunity for parenteral exposure. It seems likely that the efficiency of disease transmission is much lower in these cases. There has been no documentation of a nonparenteral transmission of HDV. Vertical transmission in utero and perinatal transmission occur with HBV, HCV, and HDV. CLASSIFICATION AND CLINICAL DISEASE The clinical illness typically found in about 90% of symptomatic patients may comprise lassitude, anorexia, weakness, nausea, dark urine, fever, vomiting, headache, chills, and abdominal discomfort, with other miscellaneous symptoms occurring somewhat less commonly. Laboratory abnormalities reflect liver cell necrosis and include primarily an elevation of alanine aminotransferase and, less commonly, elevations of levels of alkaline phosphatase and bilirubin. In most typical cases of viral hepatitis, the pathologic findings in the liver consist of combinations of portal, periportal, and lobular hepatitis. There is an accumulation of inflammatory cells and parenchymal cell necrosis throughout the liver. Differences in histopathology generally correlate with the clinical severity and resolve completely with recovery from the illness. Recovery is complete in about 85% to 90% of patients 2 to 6 weeks after the onset of illness. The course of HFV infection has been incompletely defined; it appears to be a milder acute disease than HCV infection, for example, but to have a frequency of chronicity of 10% to 29%. HBV and HCV infections, however, have variants of typical disease, representing 10% and 30%, respectively, of the clinically detectable cases. In HBV infection, manifestations may be atypical by virtue of the type of abnormalities or the severity or duration of the abnormality. As to the first possibility, extrahepatic manifestations of hepatitis B most frequently occur in the form of arthralgias, predominantly involving the small joints. Other reported immune phenomena include arteritis, nephritis, and dermatitis. Extrahepatic manifestations occur with HCV and HDV infections as well. Viral hepatitis, particularly that caused by HCV, may also present atypically with a primarily cholestatic profile of liver function abnormalities. 41 Patients with this profile may have profound jaundice and pruritus but no anatomic obstruction of the biliary system. Atypical viral hepatitis manifestations that differ in duration or severity are described as follows by the expert panel contributing to The Hepatitis Knowledge Base. 10 Prolonged viral hepatitis is defined simply as typical acute disease lasting 4 to 12 months. It differs from relapsing viral hepatitis in that the latter resolves within several months but only after an unsettling relapse or two of typical disease. Prolonged viral hepatitis is not easily distinguished from chronic persistent hepatitis, which seems to share most of its features. All three entities share the features of an ultimately self-limited disease, no demonstrated effective therapy, and no sequelae. After typical acute viral hepatitis, post-hepatitis hyperbilirubinemia (with normal liver histology) may persist as the sole abnormality, only to resolve spontaneously. Carriers of HBsAg, about whom most is known, are asymptomatic and represent less than 1% of the normal population in this country, although the estimate worldwide is over 200 million carriers. 26 Acute HBV infections result in long-term carriage of HBsAg and sometimes other markers of HBV in approximately 5% of clinically identified cases. It is not unusual to lack a history of prior infection, and the carrier may or may not be infectious. Currently available tests do not make that distinction, but several guidelines may be useful. The carrier is more likely to be infectious: the closer he or she is to the acute infection, the higher the titer of anti-HBcAg, the higher the titer of hepatitis B e antigen (HBeAg), and the more immunocompromised the carrier is. In contrast, the presence of antibodies to HBsAg or HBeAg makes the individual less likely to be infectious. From a practical point of view, all carriers should be considered potentially infectious, particularly when an invasive procedure is contemplated. Judgments about the timing for a surgical procedure are dictated primarily by the indications for operation. In elective procedures, however, when it is not possible to distinguish the true chronic carrier from an individual with incubating acute viral hepatitis, a period of observation is a worthwhile precaution to allow the circumstances to clarify. Because it has not been possible to detect HCV or its antigens conclusively and because the development of antibody seems to occur later and perhaps less reliably than with HBV, it has not been possible to ascertain the frequency of the infectious carrier of HCV or to determine the consequences of exposure. That transmission from asymptomatic carriers occurs and that disease ensues in the recipient is certain, however, in view of the frequency of posttransfusion hepatitis 2 and the frequency of the documented instances of HCV infection after an accidental needlestick. Screening procedures on patients who serve as the source of a potentially infected needlestick, for example, are controversial. Neither normal results of liver function tests nor absence of HCV antibody preclude the possibility that the individual is infectious, although the risks should be substantially reduced. Of some concern to the surgeon is the possibility that he or she may become infected and subsequently be a carrier of HBV or HCV. Although the level of risk to patients has not been conclusively defined, most authorities agree that HBV transmission from physician to patients, although documented, is a very unusual event and that common hygienic measures, not to mention scrupulous surgical technique, reduce that risk perhaps to the vanishing point. 31, 36 Because of a single instance in which another bloodborne virus, human immunodeficiency virus (HIV), was transmitted from a health care provider (a dentist) to six patients, the CDC formulated a policy, now in effect in all states, that requires self-notification of all health care providers known to be positive for HBV or HIV to the state health department. 18 Routine testing of health care providers has not been mandated, and most authorities do not recommend such a practice. Of the serious sequelae, acute fulminant viral hepatitis, chronic aggressive hepatitis, and viral hepatitis with confluent hepatic necrosis (subacute hepatic necrosis) appear to represent points on a spectrum from more to less advanced. Any of these sequelae may progress to cirrhosis with its complications. Finally, there is an association between hepatoma (hepatocellular carcinoma) and HBV and possibly HCV infection, suggesting the oncogenic potential of these viruses. For HBV infection, considerable epidemiologic, molecular, and animal evidence exists; and current thinking about the most likely mechanism is that integration of the viral DNA is subjected to mutagenic stimuli over a prolonged period, resulting in hepatocellular carcinoma. No association with carcinoma has been identified with other classes of hepatitis virus. HCV infection appears to result in chronic hepatitis even more frequently than HBV infection does. Initially, chronic hepatitis caused by HCV was considered to be more benign than that associated with HBV. 9 Subsequently, however, follow-up biopsy studies found that in as many as 30% of the originally detected cases of chronic aggressive hepatitis the patients developed cirrhosis. Because HDV does not cause disease in the absence of HBV, its contribution to chronicity is somewhat complicated. Available evidence suggests that co-infection with HBV and HDV results in a long-term course indistinguishable from that with infection with HBV alone, whereas superimposed infection of HBV with HDV results in chronic HDV infection. A worsened histopathologic picture and accelerated liver disease appear to accompany the latter scenario. In addition, vaccines have been developed for HAV. Approaches include vaccines made from live, attenuated, or inactivated virus, as well as recombinant viral vaccines. Vaccine efficacy has been demonstrated using either inactivated or attenuated vaccines, and the former may be licensed in the United States in the near future. TREATMENT No specific form of therapy other than supportive care is available for acute hepatitis. A variety of forms of treatment have been used in the treatment of chronic hepatitis, with some being more effective than others in selected patient subsets with HBV, HCV, and HDV infections. In a review of studies before 1986, no conclusively beneficial therapeutic regimen was found. 15 Subsequently, trials of interferon with or without a prior tapered course of prednisone have demonstrated reduced liver function abnormalities and viral replication for up to 9 to 12 months in as many as 50% to 60% of patients with chronic hepatitis. The addition of the prednisone taper appears beneficial. Although less well studied, benefits of these or similar regimens have been detected for chronic HCV 30 and HDV infections. 23 It is not yet clear, however, that these courses of therapy for any chronic form of hepatitis will be durably effective or practical in all patients.